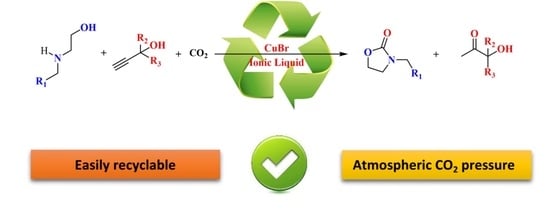
Green Synthesis of 2-Oxazolidinones by an Efficient and Recyclable CuBr/Ionic Liquid System via CO2, Propargylic Alcohols, and 2-Aminoethanols
Abstract
:1. Introduction
2. Results and Discussion
3. Investigation of the Mechanism
3.1. Activation of the Hydroxyl Group
3.2. Proposed Catalytic Mechanism
3.3. Exploration of the NHC–Cu Complexes
4. Materials and Methods
4.1. Characterization
4.2. Materials
4.3. Three-Component Reactions of Propargylic Alcohols, 2-Aminoethanols, and CO2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, M.; Jorgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Diercks, C.S.; Liu, Y.; Cordova, K.E.; Yaghi, O.M. The role of reticular chemistry in the design of CO2 reduction catalysts. Nat. Mater. 2018, 17, 301–307. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect engineering in earth-abundant electrocatalysts for CO2 and N-2 reduction. Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef] [Green Version]
- Dalpozzo, R.; Della Ca’, N.; Gabriele, B.; Mancuso, R. Recent Advances in the Chemical Fixation of Carbon Dioxide: A Green Route to Carbonylated Heterocycle Synthesis. Catalysts 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Della Ca, N.; Gabriele, B.; Ruffolo, G.; Veltri, L.; Zanetta, T.; Costa, M. Effective Guanidine-Catalyzed Synthesis of Carbonate and Carbamate Derivatives from Propargyl Alcohols in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2011, 353, 133–146. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Edit. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Lu, X.-B.; Ren, W.-M.; Wu, G.-P. CO2 Copolymers from Epoxides: Catalyst Activity, Product Selectivity, and Stereochemistry Control. Acc. Chem. Res. 2012, 45, 1721–1735. [Google Scholar] [CrossRef] [PubMed]
- Langanke, J.; Wolf, A.; Hofmann, J.; Boehm, K.; Subhani, M.A.; Mueller, T.E.; Leitner, W.; Guertler, C. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 2014, 16, 1865–1870. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Jiang, T.; He, J.; Han, B.; Wu, T.; Ding, K. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew. Chem. Int. Edit. 2007, 46, 7255–7258. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Mueller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef] [Green Version]
- Cuellar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Dindi, A.; Dang Viet, Q.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Kar, S.; Kothandaraman, J.; Goeppert, A.; Prakash, G.K.S. Advances in catalytic homogeneous hydrogenation of carbon dioxide to methanol. J. CO2 Util. 2018, 23, 212–218. [Google Scholar] [CrossRef]
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO2 utilization technology: A patent landscape review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Song, D.; Li, D.; Xiao, X.; Cheng, C.; Chaemchuen, S.; Yuan, Y.; Verpoort, F. Synthesis of beta-oxopropylcarbamates in a recyclable AgBr/ionic liquid catalytic system: An efficient assembly of CO2 under ambient pressure. J. CO2 Util. 2018, 27, 217–222. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Darabi, M.; Soroush, E.; Mesbah, M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 2019, 210, 920–926. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Wang, L.; Li, Q.; Zhang, S.; Chen, B.; Jiang, L.; Zhang, Y. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas. Appl. Energy 2019, 242, 302–310. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Olson, W.; Liang, Z. CO2 capture with hybrid absorbents of low viscosity imidazolium-based ionic liquids and amine. Appl. Energy 2019, 235, 311–319. [Google Scholar] [CrossRef]
- Rongwong, W.; Jiraratananon, R.; Archariyawut, S. Experimental study on membrane wetting in gas-liquid membrane contacting process for CO2 absorption by single and mixed absorbents. Sep. Purif. Technol. 2009, 69, 118–125. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, S.; Ebrahimiasl, S.; Arshadi, S.; Hosseinian, A. Synthesis of six-membered cyclic carbamates employing CO2 as building block: A review. J. CO2 Util. 2019, 33, 37–45. [Google Scholar] [CrossRef]
- Arshadi, S.; Vessally, E.; Sobati, M.; Hosseinian, A.; Bekhradnia, A. Chemical fixation of CO2 to N-propargylamines: A straightforward route to 2-oxazolidinones. J. CO2 Util. 2017, 19, 120–129. [Google Scholar] [CrossRef]
- Chen, F.; Li, M.; Wang, J.; Dai, B.; Liu, N. Fe(II) complexes: Reservoirs for Lewis acids and carbenes and their utility in the conversion of CO2 to oxazolidinones. J. CO2 Util. 2018, 28, 181–188. [Google Scholar] [CrossRef]
- Farshbaf, S.; Fekri, L.Z.; Nikpassand, M.; Mohammadi, R.; Vessally, E. Dehydrative condensation of beta-aminoalcohols with CO2: An environmentally benign access to 2-oxazolidinone derivatives. J. CO2 Util. 2018, 25, 194–204. [Google Scholar] [CrossRef]
- Li, X.; Ke, J.; Wang, J.; Kang, M.; Zhao, Y.; Li, Q.; Liang, C. CO2 derived amino-alcohol compounds for preparation of polyurethane adhesives. J. CO2 Util. 2019, 31, 198–206. [Google Scholar] [CrossRef]
- Li, X.; Ke, J.; Wang, J.; Liang, C.; Kang, M.; Zhao, Y.; Li, Q. A new amino-alcohol originated from carbon dioxide and its application as chain extender in the preparation of polyurethane. J. CO2 Util. 2018, 26, 52–59. [Google Scholar] [CrossRef]
- Pulla, S.; Felton, C.M.; Ramidi, P.; Gartia, Y.; Ali, N.; Nasini, U.B.; Ghosh, A. Advancements in oxazolidinone synthesis utilizing carbon dioxide as a C1 source. J. CO2 Util. 2013, 2, 49–57. [Google Scholar] [CrossRef]
- Werner, T.; Tenhumberg, N. Synthesis of cyclic carbonates from epoxides and CO2 catalyzed by potassium iodide and amino alcohols. J. CO2 Util. 2014, 7, 39–45. [Google Scholar] [CrossRef]
- Arshadi, S.; Vessally, E.; Hosseinian, A.; Soleimani-amiri, S.; Edjlali, L. Three-component coupling of CO2, propargyl alcohols, and amines: An environmentally benign access to cyclic and acyclic carbamates (A Review). J. CO2 Util. 2017, 21, 108–118. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2-Oxazolidinones. Angew. Chem. Int. Edit. 2015, 54, 5399–5403. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Song, Q.-W.; Ma, R.; Xie, J.-N.; He, L.-N. Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(II)-substituted polyoxometalate-based ionic liquids. Green Chem. 2016, 18, 282–287. [Google Scholar] [CrossRef]
- Haindl, M.H.; Hioe, J.; Gschwind, R.M. The Proline Enamine Formation Pathway Revisited in Dimethyl Sulfoxide: Rate Constants Determined via NMR. J. Am. Chem. Soc. 2015, 137, 12835–12842. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.-Y.; Wang, S.-Y.; Wang, Q.; He, L.-N. InSitu Generated Zinc(II) Catalyst for Incorporation of CO2 into 2-Oxazolidinones with Propargylic Amines at Atmospheric Pressure. ChemSusChem 2017, 10, 1210–1216. [Google Scholar] [CrossRef]
- Prasad, J.V. New oxazolidinones. Curr. Opin. Microbiol. 2007, 10, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ali, A.; Hammond, M.L.; Li, H.; Lu, Z.; Napolitano, J.; Taylor, G.E.; Thompson, C.F.; Anderson, M.S.; Chen, Y.; et al. Biphenyl-Substituted Oxazolidinones as Cholesteryl Ester Transfer Protein Inhibitors: Modifications of the Oxazolidinone Ring Leading to the Discovery of Anacetrapib. J. Med. Chem. 2011, 54, 4880–4895. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.A.; Wright, G.D. Streptogramins, oxazolidinones, and other inhibitors of bacterial protein synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Gao, S. Recent Advances in Aerobic Oxidation of Alcohols and Amines to Imines. ACS Catal. 2015, 5, 5851–5876. [Google Scholar] [CrossRef]
- Omae, I. Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord. Chem. Rev. 2012, 256, 1384–1405. [Google Scholar] [CrossRef]
- Niemi, T.; Fernandez, I.; Steadman, B.; Mannisto, J.K.; Repo, T. Carbon dioxide-based facile synthesis of cyclic carbamates from amino alcohols. Chem. Commun. 2018, 54, 3166–3169. [Google Scholar] [CrossRef] [Green Version]
- Dinsmore, C.J.; Mercer, S.P. Carboxylation and mitsunobu reaction of amines to give carbamates: Retention vs inversion of configuration is substituent-dependent. Org. Lett. 2004, 6, 2885–2888. [Google Scholar] [CrossRef]
- Juarez, R.; Concepcion, P.; Corma, A.; Garcia, H. Ceria nanoparticles as heterogeneous catalyst for CO2 fixation by omega-aminoalcohols. Chem. Commun. 2010, 46, 4181–4183. [Google Scholar] [CrossRef] [PubMed]
- Pulla, S.; Felton, C.M.; Gartia, Y.; Ramidi, P.; Ghosh, A. Synthesis of 2-Oxazolidinones by Direct Condensation of 2-Aminoalcohols with Carbon Dioxide Using Chlorostannoxanes. ACS Sustain. Chem. Eng. 2013, 1, 309–312. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; Wang, M.-Y.; Zhang, K.; Liu, P.; Xun, J.-Y.; He, L.-N. Thermodynamically Favorable Synthesis of 2-Oxazolidinones through Silver-Catalyzed Reaction of Propargylic Alcohols, CO2, and 2-Aminoethanols. ChemSusChem 2016, 9, 2054–2058. [Google Scholar] [CrossRef]
- Li, X.-D.; Song, Q.-W.; Lang, X.-D.; Chang, Y.; He, L.-N. Ag-I/TMG-Promoted Cascade Reaction of Propargyl Alcohols, Carbon Dioxide, and 2-Aminoethanols to 2-Oxazolidinones. ChemPhysChem 2017, 18, 3182–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-D.; Cao, Y.; Ma, R.; He, L.-N. Thermodynamically favorable protocol for the synthesis of 2-oxazolidinones via Cu(I)-catalyzed three-component reaction of propargylic alcohols, CO2 and 2-aminoethanols. J. CO2 Util. 2018, 25, 338–345. [Google Scholar] [CrossRef]
- Xia, S.; Song, Y.; Li, X.; Li, H.; He, L.-N. Ionic Liquid-Promoted Three-Component Domino Reaction of Propargyl Alcohols, Carbon Dioxide and 2-Aminoethanols: A Thermodynamically Favorable Synthesis of 2-Oxazolidinones. Molecules 2018, 23, 3033. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Gong, Y.; Bu, C.; Hu, J.; Zhang, Y.; Chen, C.; Chaemchuen, S.; Yuan, Y.; Verpoort, F. An efficient and recyclable AgNO3/ionic liquid system catalyzed atmospheric CO2 utilization: Simultaneous synthesis of 2-oxazolidinones and α-hydroxyl ketones. J. Catal. 2021, 393, 70–82. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into alpha-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Chen, K.; Shi, G.; Dao, R.; Mei, K.; Zhou, X.; Li, H.; Wang, C. Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Commun. 2016, 52, 7830–7833. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Yoshida, S.; Sugawara, Y.; Yamada, W.; Cheng, H.-M.; Fukui, K.; Sekine, K.; Iwakura, I.; Ikeno, T.; Yamada, T. Silver-Catalyzed Carbon Dioxide Incorporation and Rearrangement on Propargylic Derivatives. Bull. Chem. Soc. Jpn. 2011, 84, 698–717. [Google Scholar] [CrossRef]
- Yamada, W.; Sugawara, Y.; Cheng, H.M.; Ikeno, T.; Yamada, T. Silver-catalyzed incorporation of carbon dioxide into propargylic alcohols. Eur. J. Org. Chem. 2007, 2007, 2604–2607. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Zeng, C.; Song, D.; Chaemchuen, S.; Chen, C.; Verpoort, F. A simple and robust AgI/KOAc catalytic system for the carboxylative assembly of propargyl alcohols and carbon dioxide at atmospheric pressure. Catal. Sci. Technol. 2017, 7, 2935–2939. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Zeng, C.; Song, D.; Chaemchuen, S.; Chen, C.; Verpoort, F. A recyclable AgI/OAc- catalytic system for the efficient synthesis of alpha-alkylidene cyclic carbonates: Carbon dioxide conversion at atmospheric pressure. Green Chem. 2017, 19, 2936–2940. [Google Scholar] [CrossRef]
- Li, M.; Abdolmohammadi, S.; Hoseininezhad-Namin, M.S.; Behmagham, F.; Vessally, E. Carboxylative cyclization of propargylic alcohols with carbon dioxide: A facile and Green route to alpha-methylene cyclic carbonates. J. CO2 Util. 2020, 38, 220–231. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, Y.; Song, D.; Zeng, C.; Chaemchuen, S.; Chen, C.; Verpoort, F. One-pot carboxylative cyclization of propargylic alcohols and CO2 catalysed by N-heterocyclic carbene/Ag systems. Appl. Organomet. Chem. 2017, 31, e3867. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Du, M.; Bu, C.; Chen, C.; Chaemcheun, S.; Hu, J.; Zhang, Y.; Yuan, Y.; Verpoort, F. CO2-Promoted Hydration of Propargylic Alcohols: Green Synthesis of alpha-Hydroxy Ketones by an Efficient and Recyclable AgOAc/lonic Liquid System. ACS Sustain. Chem. Eng. 2020, 8, 8148–8155. [Google Scholar] [CrossRef]
- Steckel, J.A. Ab Initio Calculations of the Interaction between CO2 and the Acetate Ion. J. Phys. Chem. A 2012, 116, 11643–11650. [Google Scholar] [CrossRef]
- Wang, W.-H.; Feng, X.; Sui, K.; Fang, D.; Bao, M. Transition metal-free carboxylation of terminal alkynes with carbon dioxide through dual activation: Synthesis of propiolic acids. J. CO2 Util. 2019, 32, 140–145. [Google Scholar] [CrossRef]





 | ||||
|---|---|---|---|---|
| Entry | [Cu] Salt | Ionic Liquid | Yield (%) b | |
| 3a b | 4a b | |||
| 1 | ‒ | [C2C1im][OAc] | 0 | 0 |
| 2 | CuBr | ‒ | 0 | 0 |
| 3 | ‒ | ‒ | 0 | 0 |
| 4 | CuBr | [C2C1im][OAc] | 59 | 55 |
| 5 | CuCl | [C2C1im][OAc] | 56 | 50 |
| 6 | CuI | [C2C1im][OAc] | 55 | 56 |
| 7 | Cu2S | [C2C1im][OAc] | 22 | 18 |
| 8 | Cu(CH3CN)4PF6 | [C2C1im][OAc] | 28 | 30 |
| 9 | C4H3S-COO-Cu | [C2C1im][OAc] | 55 | 57 |
| 10 | CuSCN | [C2C1im][OAc] | 36 | 30 |
| 11 | CuOAc | [C2C1im][OAc] | 17 | 14 |
| 12 | CuBr | [C2C1im][ClO4] | 0 | 0 |
| 13 | CuBr | [C2C1im]I | 0 | 0 |
| 14 | CuBr | [C2C1im][BF4] | 0 | 0 |
| 15 | CuBr | [C2C1im][PF6] | 0 | 0 |
| 16 | CuBr | [C2C1im][OTf] | 0 | 0 |
| 17 | CuBr | [C2C1im]Br | 20 | 24 |
| 18 | CuBr | [C2C1im][NO3] | 24 | 27 |
| 19 | CuBr | [C4C1im][OAc] | 60 | 60 |
| 20 | CuBr | [N4444][OAc] | 48 | 39 |
| 21 | CuBr | [DBUH][OAc] | 37 | 35 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | CuBr (mol%) | [C4C1im][OAc] (mmol) | Temperature (°C) | The Ratio of 1a:2a | Yield (%) b | |
| 3a b | 4a b | |||||
| 1 | 0.5 | 6.5 | 25 | 1:1.5 | 0 | 0 |
| 2 | 0.5 | 6.5 | 50 | 1:1.5 | 0 | 0 |
| 3 | 0.5 | 6.5 | 80 | 1:1.5 | 60 | 60 |
| 4 | 0.5 | 6.5 | 100 | 1:1.5 | 92 | 96 |
| 5 | 0.5 | 6.5 | 120 | 1:1.5 | 90 | 95 |
| 6 | 0.5 | 3.2 | 80 | 1:1.5 | 27 | 27 |
| 7 | 0.5 | 13 | 80 | 1:1.5 | 55 | 58 |
| 8 | 0.25 | 6.5 | 80 | 1:1.5 | 20 | 23 |
| 9 | 0.5 | 6.5 | 80 | 1:1 | 44 | 48 |
| 10 c | 0.5 | 6.5 | 100 | 1:1.5 | 40 | 43 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Product (Yield/%) b | ||||
| 1 | 2 | 3 | 4 | |||
| 1 |  1a |  2a |  3a | 92 90 c 83 d |  4a | 96 93 c 78 d |
| 2 |  1a |  2b |  3a | 84 |  4b | 90 |
| 3 |  1a |  2c |  3a | 86 80 d |  4c | 85 79 d |
| 4 |  1a |  2d |  3a | 94 e |  4d | 98 e |
| 5 |  1a |  2e |  3a | 84 f 79 d |  4e | 85 f 80 d |
| 6 |  1b |  2a |  3b | 87 80 d |  4a | 91 77 d |
| 7 |  1c |  2a |  3c | 83 e |  4a | 89 e |
| 8 |  1d |  2a |  3d | 90 e |  4a | 89 e |
| 9 |  1e |  2a |  3e | 85 |  4a | 90 |
| 10 |  1f |  2a |  3f | 17 e |  4a | 23 e |
| 11 |  1g |  2a |  3g | 51 g |  4a | 50 g |
| 12 |  1h |  2a |  3h | 77 e |  4a | 80 e |
| 13 |  1i |  2a |  3i | 73 e |  4a | 76 e |
| 14 |  1j |  2a |  3j | 92 e |  4a | 93 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, C.; Gong, Y.; Du, M.; Chen, C.; Chaemchuen, S.; Hu, J.; Zhang, Y.; Díaz Velázquez, H.; Yuan, Y.; Verpoort, F. Green Synthesis of 2-Oxazolidinones by an Efficient and Recyclable CuBr/Ionic Liquid System via CO2, Propargylic Alcohols, and 2-Aminoethanols. Catalysts 2021, 11, 233. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020233
Bu C, Gong Y, Du M, Chen C, Chaemchuen S, Hu J, Zhang Y, Díaz Velázquez H, Yuan Y, Verpoort F. Green Synthesis of 2-Oxazolidinones by an Efficient and Recyclable CuBr/Ionic Liquid System via CO2, Propargylic Alcohols, and 2-Aminoethanols. Catalysts. 2021; 11(2):233. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020233
Chicago/Turabian StyleBu, Chao, Yanyan Gong, Minchen Du, Cheng Chen, Somboon Chaemchuen, Jia Hu, Yongxing Zhang, Heriberto Díaz Velázquez, Ye Yuan, and Francis Verpoort. 2021. "Green Synthesis of 2-Oxazolidinones by an Efficient and Recyclable CuBr/Ionic Liquid System via CO2, Propargylic Alcohols, and 2-Aminoethanols" Catalysts 11, no. 2: 233. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020233