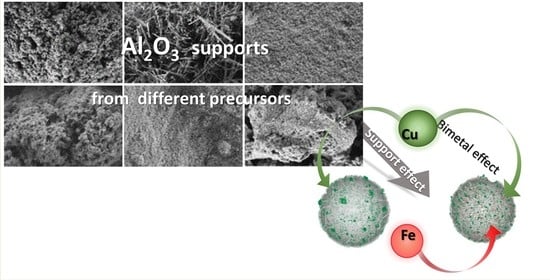
Influence of Alumina Precursor Properties on Cu-Fe Alumina Supported Catalysts for Total Toluene Oxidation as a Model Volatile Organic Air Pollutant
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Catalysts Presursors and Catalysts
2.1.1. X-ray Diffraction
2.1.2. SEM, Elemental Analysis, N2 Physisorption and Pyridine TPD
2.1.3. UV-Vis DR and FTIR Spectroscopies
2.1.4. Al NMR Spectroscopy
2.1.5. Temperature Programed Reduction (H2-TPR)
2.1.6. Transmission Electron Microscopy (TEM)
2.2. Catalytic Properties of the Catalysts
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
4.1.1. Materials
4.1.2. Synthesis
4.2. Catalyst Characterization
4.3. Catalytic Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. European Topic Centre on Air Pollution and Climate Change Mitigation (ETC/ACM) Air Quality in Europe: 2017 Report; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-9213-921-6. [Google Scholar]
- Tomatis, M.; Xu, H.-H.; He, J.; Zhang, X.-D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Niskala Koivikko, N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224–1256. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low Temperature Catalytic Oxidation of Volatile Organic Compounds: A Review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Chen, Z.W.; Chen, L.X.; Yang, C.C.; Jiang, Q. Atomic (Single, Double, and Triple Atoms) Catalysis: Frontiers, Opportunities, and Challenges. J. Mater. Chem. A 2019, 7, 3492–3515. [Google Scholar] [CrossRef]
- Liu, W.-J.; Qian, T.-T.; Jiang, H. Bimetallic Fe Nanoparticles: Recent Advances in Synthesis and Application in Catalytic Elimination of Environmental Pollutants. Chem. Eng. J. 2014, 236, 448–463. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Volatile Organic Compounds Abatement over Copper-Based Catalysts: Effect of Support. Inorg. Chim. Acta 2017, 455, 473–482. [Google Scholar] [CrossRef]
- Popova, M.; Ristić, A.; Lazar, K.; Maučec, D.; Vassileva, M.; NovakTušar, N. Iron-Functionalized Silica Nanoparticles as a Highly Efficient Adsorbent and Catalyst for Toluene Oxidation in the Gas Phase. ChemCatChem 2013, 5, 986–993. [Google Scholar] [CrossRef]
- Djinović, P.; Ristić, A.; Žumbar, T.; Dasireddy, V.D.B.C.; Rangus, M.; Dražić, G.; Popova, M.; Likozar, B.; Zabukovec Logar, N.; Novak Tušar, N. Synergistic Effect of CuO Nanocrystals and Cu-Oxo-Fe Clusters on Silica Support in Promotion of Total Catalytic Oxidation of Toluene as a Model Volatile Organic Air Pollutant. Appl. Catal. B Environ. 2020, 268, 118749. [Google Scholar] [CrossRef]
- Taylor, S.H.; Heneghan, C.S.; Hutchings, G.J.; Hudson, I.D. The Activity and Mechanism of Uranium Oxide Catalysts for the Oxidative Destruction of Volatile Organic Compounds. Catal. Today 2000, 59, 249–259. [Google Scholar] [CrossRef]
- Antunes, A.P.; Ribeiro, M.F.; Silva, J.M.; Ribeiro, F.R.; Magnoux, P.; Guisnet, M. Catalytic Oxidation of Toluene over CuNaHY Zeolites: Coke Formation and Removal. Appl. Catal. B Environ. 2001, 33, 149–164. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Xia, Q.; Liu, Z.; Li, Z. Catalytic Oxidation of Toluene over Copper and Manganese Based Catalysts: Effect of Water Vapor. Catal. Commun. 2011, 14, 15–19. [Google Scholar] [CrossRef]
- Xue, T.; Li, R.; Gao, W.; Gao, Y.; Wang, Q.; Umar, A. Preparation and Characterization of Highly Efficient CuFe Mixed Oxides for Total Oxidation of Toluene. J. Nanosci. Nanotechnol. 2018, 18, 3381–3386. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.-K.; Nah, J.W. Property of a Highly Active Bimetallic Catalyst Based on a Supported Manganese Oxide for the Complete Oxidation of Toluene. Powder Technol. 2014, 266, 292–298. [Google Scholar] [CrossRef]
- Wang, C.-H. Al2O3-Supported Transition-Metal Oxide Catalysts for Catalytic Incineration of Toluene. Chemosphere 2004, 55, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilirad, M.; Zabihi, M.; Shayegan, J.; Khorasheh, F. Oxidation of Toluene in Humid Air by Metal Oxides Supported on γ-Alumina. J. Hazard. Mater. 2017, 333, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białas, A.; Kuśtrowski, P.; Dudek, B.; Piwowarska, Z.; Wach, A.; Michalik, M.; Kozak, M. Copper-Aluminum Oxide Catalysts for Total Oxidation of Toluene Synthesized by Thermal Decomposition of Co-Precipitated Precursors. Thermochim. Acta 2014, 590, 191–197. [Google Scholar] [CrossRef]
- Downs, A.J. Chemistry of Aluminium, Gallium, Indium and Thallium; Springer Science & Business Media: Glasgow, UK, 1993; Volume 111, ISBN 978-0-7514-0103-5. [Google Scholar]
- Busca, G. Structural, Surface, and Catalytic Properties of Aluminas. Adv. Catal. 2014, 57, 319–404. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Busca, G. Catalytic Materials Based on Silica and Alumina: Structural Features and Generation of Surface Acidity. Prog. Mater. Sci. 2019, 104, 215–249. [Google Scholar] [CrossRef]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Laboratories: Pittsburg, PA, USA, 1987. [Google Scholar]
- Walker, G.S.; Pyke, D.R.; Werrett, C.R.; Williams, E.; Bhattacharya, A.K. Surface Reactivity of Aluminas Prepared by Different Techniques. Appl. Surf. Sci. 1999, 1999, 228–234. [Google Scholar] [CrossRef]
- Lafficher, R.; Digne, M.; Salvatori, F.; Boualleg, M.; Colson, D.; Puel, F. Development of New Alumina Precipitation Routes for Catalysis Applications. J. Cryst. Growth 2017, 468, 526–530. [Google Scholar] [CrossRef]
- Stoica, G.; Pérez-Ramírez, J. Institut Català d’Investigació Química Chemistry of Dawsonites and Application in Catalysis Doctoral Thesis; Universitat Rovira i Virgili: Tarragona, Spain, 2010; ISBN 978-84-693-1533-0. [Google Scholar]
- Ali, A.A.; Hasan, M.A.; Zaki, M.I. Dawsonite-Type Precursors for Catalytic Al, Cr, and Fe Oxides: Synthesis and Characterization. Chem. Mater. 2005, 17, 6797–6804. [Google Scholar] [CrossRef]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.M.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.W.; Jung, K.D.; Lee, K.Y.; Yoo, K.S. Influence of Structure Type of Al2O3 on Dehydration of Methanol for Dimethyl Ether Synthesis. Ind. Eng. Chem. Res. 2008, 47, 6573–6578. [Google Scholar] [CrossRef]
- Laurenti, D.; Phung-Ngoc, B.; Roukoss, C.; Devers, E.; Marchand, K.; Massin, L.; Lemaitre, L.; Legens, C.; Quoineaud, A.-A.; Vrinat, M. Intrinsic Potential of Alumina-Supported CoMo Catalysts in HDS: Comparison between Γc, ΓT, and δ-Alumina. J. Catal. 2013, 297, 165–175. [Google Scholar] [CrossRef]
- Louw, L.-A. The Synthesis of Aluminium Hydroxide and Oxyhydroxide. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1993. [Google Scholar]
- Lefèvre, G.; Duc, M.; Lepeut, P.; Caplain, R.; Fédoroff, M. Hydration of γ-Alumina in Water and Its Effects on Surface Reactivity. Langmuir 2002, 18, 7530–7537. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Kocaefe, Y.; Cheng, J.; Liu, W. The Effect of Novel Synthetic Methods and Parameters Control on Morphology of Nano-Alumina Particles. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and Reactivity of Framework and Extra-Framework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Dziembaj, R.; Cool, P.; Vansant, E.F. Catalytic Performance of Various Mesoporous Silicas Modified with Copper or Iron Oxides Introduced by Different Ways in the Selective Reduction of NO by Ammonia. Appl. Catal. B Environ. 2006, 62, 369–380. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, P.; Ding, D.; Yang, Z.; Wang, W.; Xin, H.; Xu, J.; Han, Y.-F. Revealing the Active Species of Cu-Based Catalysts for Heterogeneous Fenton Reaction. Appl. Catal. B Environ. 2019, 258, 117985. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Santhosh Kumar, M.; Brückner, A. Reduction of N2O with CO over FeMFI Zeolites: Influence of the Preparation Method on the Iron Species and Catalytic Behavior. J. Catal. 2004, 223, 13–27. [Google Scholar] [CrossRef]
- Xin, Q.; Glisenti, A.; Philippopoulos, C.; Poulakis, E.; Mertens, M.; Nyalosaso, J.; Meynen, V.; Cool, P. Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement. Catalysts 2016, 6, 164. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, C.; Bordiga, S.; Salvalaggio, M.; Spoto, G.; Zecchina, A.; Geobaldo, F.; Vlaic, G.; Bellatreccia, M. XAFS, IR, and UV- Vis Study of the CuI Environment in CuI-ZSM-5. J. Phys. Chem. B 1997, 101, 344–360. [Google Scholar] [CrossRef]
- Taifan, W.E.; Li, Y.; Baltrus, J.P.; Zhang, L.; Frenkel, A.I.; Baltrusaitis, J. Operando Structure Determination of Cu and Zn on Supported MgO/SiO2 Catalysts during Ethanol Conversion to 1,3-Butadiene. ACS Catal. 2019, 9, 269–285. [Google Scholar] [CrossRef]
- Acikgoz, M.; Khoshi, M.R.; Harrell, J.; Genova, A.; Chawla, R.; He, H.; Pavanello, M. Tuning the Electronic Properties of the γ-Al2O3 Surface by Phosphorus Doping. Phys. Chem. Chem. Phys. 2019, 21, 15080–15088. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition Alumina Phases Induced by Heat Treatment of Boehmite: An X-ray Diffraction and Infrared Spectroscopy Study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tang, J.Y.; Wang, G.L.; Zhang, M.; Hu, X.Y. Facile Synthesis of Submicron Cu2O and CuO Crystallites from a Solid Metallorganic Molecular Precursor. J. Cryst. Growth 2006, 294, 278–282. [Google Scholar] [CrossRef]
- Stoica, G.; Abelló, S.; Pérez-Ramírez, J. Na-Dawsonite Derived Aluminates for DMC Production by Transesterification of Ethylene Carbonate. Appl. Catal. Gen. 2009, 365, 252–260. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schuchardt, U. On the Paradox of Transition Metal-Free Alumina-Catalyzed Epoxidation with Aqueous Hydrogen Peroxide. J. Catal. 2005, 236, 335–345. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Javier Garcia-Garcia, F.; Häussermann, U. Structural Analysis of Highly Porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Chandran, C.V.; Kirschhock, C.E.A.; Radhakrishnan, S.; Taulelle, F.; Martens, J.A.; Breynaert, E. Alumina: Discriminative Analysis Using 3D Correlation of Solid-State NMR Parameters. Chem. Soc. Rev. 2019, 48, 134–156. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.J.; Kwak, J.H. Acid-Base Properties of Al2O3: Effects of Morphology, Crystalline Phase, and Additives. J. Catal. 2017, 345, 135–148. [Google Scholar] [CrossRef]
















| Sample Abbreviation | SBET (m2/g) | VP (cm3/g) | dP (nm) | cA.S. (mmol/g) | cA.S. (μmol/m2) | c350 (%) | c410 (%) |
|---|---|---|---|---|---|---|---|
| N-FeCu | 180 | 0.349 | 4–6 | 0.3096 | 1.72 | 34 | 77 |
| N-Cu | 171 | 0.308 | 4–6 | 0.2272 | 1.32 | 37 | 77 |
| N-Fe-500 | 229 | 0.338 | 5 | 0.1554 | 0.68 | 13 | 19 |
| N-500 | 221 | 0.353 | 5 | 0.1764 | 0.80 | / | / |
| Sample Abbreviation | Precursor Structure | SBET (m2/g) | VP (cm3/g) | dP (nm) | cA.S. (μmol/g) | cA.S. (μmol/m2) | c350 (%) | c410 (%) |
|---|---|---|---|---|---|---|---|---|
| a-FeCu | Amorphous | 45 | 0.102 | >80 | 136 | 3.01 | 28 | 32 |
| d-FeCu | Dawsonite | 26 | 0.103 | / 1 | 47 | 1.81 | 16 | 22 |
| N-FeCu | Bayerite | 180 | 0.349 | 4–6 | 310 | 1.72 | 34 | 77 |
| p-N-FeCu | Pseudoboehmite | 286 | 1.110 | 13–63 | 454 | 1.59 | 42 | 78 |
| p-AS-FeCu | Pseudoboehmite | 178 | 0.549 | 19; >80 | 239 | 1.34 | 38 | 80 |
| b-O-FeCu | Bayerite | 225 | 0.274 | 3 | 324 | 1.41 | 34 | 84 |
| Sample Abbreviation | Preparation Difference | Precursor Structure | CuO (wt%) | Fe2O3 (wt%) | Fe/Al Molar Ratio | Cu/Al Molar Ratio |
|---|---|---|---|---|---|---|
| a-FeCu | / | Amorphous | 7.32 | 0.539 | 0.004 | 0.056 |
| d-FeCu | / | Dawsonite | 7.67 | 0.740 | 0.007 | 0.069 |
| N-FeCu | Nitric acid | Bayerite | 9.17 | 0.558 | 0.004 | 0.066 |
| p-N-FeCu | Nitric acid, without HT 1 | Pseudoboehmite | 8.00 | 1.010 | 0.008 | 0.063 |
| p-AS-FeCu | Al salts, HT 1 | Pseudoboehmite | 6.42 | 0.551 | 0.004 | 0.045 |
| b-O-FeCu | Organic acid | Bayerite | 6.81 | 0.854 | 0.004 | 0.056 |
| Sample Abbreviation | Precursor Structure | CuO (wt%) | Fe2O3 (wt%) | Fe/Al Molar Ratio | Cu/Al Molar Ratio |
|---|---|---|---|---|---|
| N-FeCu | Bayerite | 9.17 | 0.556 | 0.004 | 0.066 |
| N-Cu | Bayerite | 9.56 | 0.056 | 0.000 | 0.069 |
| N-Fe-500 | Bayerite | 0.01 | 0.512 | 0.003 | 0.000 |
| N-500 | Bayerite | 0.00 | 0.000 | 0.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žumbar, T.; Ristić, A.; Dražić, G.; Lazarova, H.; Volavšek, J.; Pintar, A.; Zabukovec Logar, N.; Tušar, N.N. Influence of Alumina Precursor Properties on Cu-Fe Alumina Supported Catalysts for Total Toluene Oxidation as a Model Volatile Organic Air Pollutant. Catalysts 2021, 11, 252. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020252
Žumbar T, Ristić A, Dražić G, Lazarova H, Volavšek J, Pintar A, Zabukovec Logar N, Tušar NN. Influence of Alumina Precursor Properties on Cu-Fe Alumina Supported Catalysts for Total Toluene Oxidation as a Model Volatile Organic Air Pollutant. Catalysts. 2021; 11(2):252. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020252
Chicago/Turabian StyleŽumbar, Tadej, Alenka Ristić, Goran Dražić, Hristina Lazarova, Janez Volavšek, Albin Pintar, Nataša Zabukovec Logar, and Nataša Novak Tušar. 2021. "Influence of Alumina Precursor Properties on Cu-Fe Alumina Supported Catalysts for Total Toluene Oxidation as a Model Volatile Organic Air Pollutant" Catalysts 11, no. 2: 252. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020252