Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation
Abstract
:1. Introduction
2. Advanced Oxidation Process
2.1. Semiconductor Nanomaterials as Photocatalyst
2.1.1. Titanium Dioxide (TiO2)
2.1.2. Zinc Oxide (ZnO)
2.1.3. Bismuth Ferrite (BiFeO3)
2.1.4. Other Metal Oxides
3. Reduced Graphene Oxide
3.1. Hybridization of rGO within Semiconductor Nanomaterials
3.1.1. Strategies and Performance of Hybridization rGO-Based Photocatalyst
Hydrothermal Method
Sol-gel Method
Ultrasonication Method
Wet Impregnation and Co-Precipitation Method
Electrophoretic Deposition, Electrospinning and Ultrasonic Spray Analysis Methods
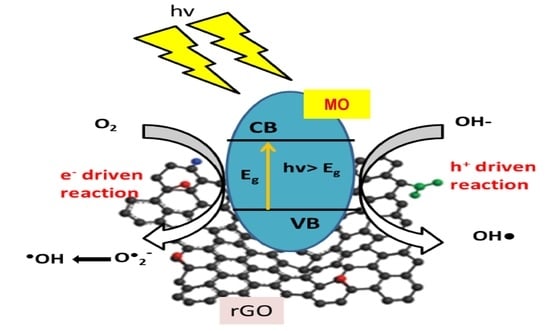
4. Mechanism of Photocatalytic Degradation of Organic Pollutants by rGO-Based Nanocomposites
Scavenger’s Test and Kinetics Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunca, A.-M. Water pollution and water quality assessment of major transboundary rivers from Banat (Romania). J. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Milanović, A.; Milijašević, D.; Brankov, J. Assessment of polluting effects and surface water quality using water pollution index: A case study of Hydro-system Danube-Tisa-Danube, Serbia. Carpathian J. Earth Environ. Sci. 2011, 6, 269–277. [Google Scholar]
- Chaudhary, M.; Walker, T.R. River Ganga pollution: Causes and failed management plans (correspondence on Dwivedi et al. 2018. Ganga water pollution: A potential health threat to inhabitants of Ganga basin. Environment International 117, 327–338). Environ. Int. 2019, 126, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Li, Q.; Hu, H.; Peng, F.; Zhang, P.; Li, J. Spatial characteristics and influencing factors of river pollution in China. Water Environ. Res. 2019, 91, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Rashid, M.Z.M.; Nasir, F.A.M.; Yee, W.S.; Aris, A.Z. Occurrence and potential human health risk of pharmaceutical residues in drinking water from Putrajaya (Malaysia). Ecotoxicol. Environ. Saf. 2019, 180, 549–556. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, K.; Lu, P.; Zhang, D. Facile fabrication of sandwich-like BiOI/AgI/g-C3N4 composites for efficient photocatalytic degradation of methyl orange and reduction of Cr(VI). J. Nanoparticle Res. 2018, 20, 328. [Google Scholar] [CrossRef]
- Walter, M.V.; Vennes, J.W. Occurrence of multiple-antibiotic-resistant enteric bacteria in domestic sewage and oxidation lagoons. Appl. Environ. Microbiol. 1985, 50, 930–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, G.; Kaur, A.; Sinha, A.S.K.; Kansal, S.K. Photocatalytic degradation of levofloxacin in aqueous phase using Ag/AgBr/BiOBr microplates under visible light. Mater. Res Bull. 2017, 88, 148–155. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.; Michael, C.; Xekoukoulotakis, N.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef]
- Jones, K.C.; De Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Jiang, J.-Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Emerging Compounds Removal from Wastewater. Green Chem. Sustain. Published online. 2012, 15–38. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. A review on the visible light active BiFeO3 nanostructures as suitable photocatalyst in the degradation of different textile dyes. Environ. Nanotechnol. Monit. Manag. 2017, 7, 110–120. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Isari, A.A.; Fattahi, M.; Anvaripour, B.; Jorfi, S. Photocatalytic decontamination of phenol and petrochemical wastewater through ZnO/TiO2 decorated on reduced graphene oxide nanocomposite: Influential operating factors, mechanism, and electrical energy consumption. RSC Adv. 2018, 8, 40035–40053. [Google Scholar] [CrossRef] [Green Version]
- Lafi, W.K.; Shannak, B.; Al-Shannag, M.; Al-Anber, Z.; Al-Hasan, M. Treatment of olive mill wastewater by combined advanced oxidation and biodegradation. Sep. Purif. Technol. 2009, 70, 141–146. [Google Scholar] [CrossRef]
- Huang, C.; Dong, C.; Tang, Z. Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Wammer, K.H.; Korte, A.R.; Lundeen, R.A.; Sundberg, J.E.; McNeill, K.; Arnold, W.A. Direct photochemistry of three fluoroquinolone antibacterials: Norfloxacin, ofloxacin, and enrofloxacin. Water Res. 2013, 47, 439–448. [Google Scholar] [CrossRef] [PubMed]
- De Bel, E.; Dewulf, J.; De Witte, B.; Van Langenhove, H.; Janssen, C. Influence of pH on the sonolysis of ciprofloxacin: Biodegradability, ecotoxicity and antibiotic activity of its degradation products. Chemosphere 2009, 77, 291–295. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Fattakassinos, D. Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: Evaluation of operational and kinetic parameters. Water Res. 2010, 44, 5450–5462. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Liu, J.; Yan, X.; Oh, W.-D.; Lim, T.-T. Low-temperature synthesis of graphene/Bi2Fe4O9 composite for synergistic adsorption-photocatalytic degradation of hydrophobic pollutant under solar irradiation. Chem. Eng. J. 2015, 262, 1022–1032. [Google Scholar] [CrossRef]
- Zhu, M.; Zhai, C.; Fujitsuka, M.; Majima, T. Noble metal-free near-infrared-driven photocatalyst for hydrogen production based on 2D hybrid of black Phosphorus/WS2. Appl. Catal. B Environ. 2018, 221, 645–651. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, X.; Xu, X.; Chen, X. Facile fabrication of highly efficient AgI/ZnO heterojunction and its application of methylene blue and rhodamine B solutions degradation under natural sunlight. Appl. Surf. Sci. 2014, 321, 10–18. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Ling, H.; Qiu, Y.; Lou, J.; Hou, X.; Bag, S.P.; Wang, J.; Wu, H.; Chai, G. Significant Enhancement of the visible light photocatalytic properties in 3D BiFeO3/graphene composites. Nanomaterials 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, T.; Tayyebi, A.; Lee, B.-K. Photolysis and photocatalysis of tetracycline by sonochemically heterojunctioned BiVO4/reduced graphene oxide under visible-light irradiation. J. Environ. Manag. 2019, 232, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M. Nanocomposites of SnO2 and g-C3N4: Preparation, characterization and photocatalysis under visible LED irradiation. Ceram. Int. 2018, 44, 3837–3846. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, B.; Ojha, A.K.; Das, J.; Kumar, A. Facile synthesis of CdO nanorods and exploiting its properties towards supercapacitor electrode materials and low power UV irradiation driven photocatalysis against methylene blue dye. Mater. Res. Bull. 2017, 90, 224–231. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.-M.; Sin, J.-C.; Zeng, H. Boosting visible light photocatalytic and antibacterial performance by decoration of silver on magnetic spindle-like bismuth ferrite. Mater. Sci. Semicond. Process. 2019, 101, 103–115. [Google Scholar] [CrossRef]
- Khalil, M.; Anggraeni, E.S.; Ivandini, T.A.; Budianto, E. Exposing TiO2 (001) crystal facet in nano Au-TiO2 heterostructures for enhanced photodegradation of methylene blue. Appl. Surf. Sci. 2019, 487, 1376–1384. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrog. Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Mohamed, H.H. Sonochemical synthesis of ZnO hollow microstructure/reduced graphene oxide for enhanced sunlight photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. A Chem. 2018, 353, 401–408. [Google Scholar] [CrossRef]
- Saravanakkumar, D.; Oualid, H.A.; Brahmi, Y.; Ayeshamariam, A.; Karunanaithy, M.; Saleem, A.M.; Kaviyarasu, K.; Sivaranjani, S.; Jayachandran, M. Synthesis and characterization of CuO/ZnO/CNTs thin films on copper substrate and its photocatalytic applications. OpenNano 2019, 4, 100025. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Sivakumar, B.; Kulangara, R.V.; Subramanian, B. Visible light driven photocatalytic efficiency of rGO-Ag-BiFeO3 ternary nanohybrids on the decontamination of dye-polluted water: An amalgamation of 1D, 2D and 3D systems. ChemistrySelect 2016, 1, 6961–6971. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Rao, Y.; Li, X.; Yuan, D.; Tang, S.; Zhao, Q. Enhanced photocatalytic activity of TiO2 with acetylene black and persulfate for degradation of tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 384, 123350. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Mir, N.A.; Qutub, N.; Mehraj, O.; Sabir, S.; Muneer, M. Synthesis, characterization and optimization of photocatalytic activity of TiO2/ZrO2 nanocomposite heterostructures. Mater. Sci. Eng. B 2015, 193, 137–145. [Google Scholar] [CrossRef]
- Guesh, K.; Mayoral, Á.; Márquez-Álvarez, C.; Chebude, Y.; Díaz, I. Enhanced photocatalytic activity of TiO2 supported on zeolites tested in real wastewaters from the textile industry of Ethiopia. Microporous Mesoporous Mater. 2016, 225, 88–97. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Jing, T.; Huang, B.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y. Enhancing visible light photocatalytic activity of TiO2 using a colorless molecule (2-methoxyethanol) due to hydrogen bond effect. Appl. Catal. B: Environ. 2017, 200, 230–236. [Google Scholar] [CrossRef]
- Dorraj, M.; Goh, B.T.; Sairi, N.A.; Woi, P.M.; Basirun, W.J. Improved visible-light photocatalytic activity of TiO2 co-doped with copper and iodine. Appl. Surf. Sci. 2018, 439, 999–1009. [Google Scholar] [CrossRef]
- Ghugal, S.G.; Umare, S.S.; Sasikala, R. Enhanced photocatalytic activity of TiO2 assisted by Nb, N and S multidopants. Mater. Res. Bull. 2015, 61, 298–305. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrog. Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceumL.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Vaizoğullar, A.I. TiO2/ZnO supported on sepiolite: Preparation, structural characterization, and photocatalytic degradation of flumequine antibiotic in aqueous solution. Chem. Eng. Commun. 2017, 204, 689–697. [Google Scholar] [CrossRef]
- Velanganni, S.; Pravinraj, S.; Immanuel, P.; Thiruneelakandan, R. Nanostructure CdS/ZnO heterojunction configuration for photocatalytic degradation of Methylene blue. Phys. B: Condens. Matter 2018, 534, 56–62. [Google Scholar] [CrossRef]
- Ye, J.; Li, X.; Hong, J.; Chen, J.; Fan, Q. Photocatalytic degradation of phenol over ZnO nanosheets immobilized on montmorillonite. Mater. Sci. Semicond. Process. 2015, 39, 17–22. [Google Scholar] [CrossRef]
- Dumitru, R.; Ianculescu, A.; Păcurariu, C.; Lupa, L.; Pop, A.; Vasile, B.; Surdu, A.; Manea, F. BiFeO3-synthesis, characterization and its photocatalytic activity towards doxorubicin degradation from water. Ceram. Int. 2019, 45, 2789–2802. [Google Scholar] [CrossRef]
- Si, Y.-H.; Xia, Y.; Shang, S.-K.; Xiong, X.-B.; Zeng, X.-R.; Zhou, J.; Li, Y.-Y. Enhanced visible light driven photocatalytic behavior of BiFeO3/reduced graphene oxide composites. Nanomaterials 2018, 8, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Sun, H.; Liu, X.; Sui, H.; Zhang, Y.; Zhou, D.; Guo, Q.; Ruan, Y. Enhanced photocatalytic performance of Bi2 Fe4 O9/graphene via modifying graphene composite. J. Am. Ceram. Soc. 2017, 100, 3540–3549. [Google Scholar] [CrossRef]
- Basith, M.A.; Ahsan, R.; Zarin, I.; Jalil, M.A. Enhanced photocatalytic dye degradation and hydrogen production ability of Bi25FeO40-rGO nanocomposite and mechanism insight. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, X.; Zou, Y.; Xu, Y.-H.; Pan, C.-L.; Hu, J.-S.; Hou, C.-M. Hydrothermal synthesis, influencing factors and excellent photocatalytic performance of novel nanoparticle-assembled Bi25FeO40tetrahedrons. CrystEngComm 2015, 17, 6527–6537. [Google Scholar] [CrossRef]
- Malathi, A.; Arunachalam, P.; Kirankumar, V.; Madhavan, J.; Al-Mayouf, A.M. An efficient visible light driven bismuth ferrite incorporated bismuth oxyiodide (BiFeO3/BiOI) composite photocatalytic material for degradation of pollutants. Opt. Mater. 2018, 84, 227–235. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Mansoor, Q.; Ismail, M.; Mehmood, F.; Ajmal, M.; Abid, Z. Graphene nanoplatelets induced tailoring in photocatalytic activity and antibacterial characteristics of MgO/graphene nanoplatelets nanocomposites. J. Appl. Phys. 2017, 121, 024901. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Hooda, D.; Yadav, R.; Meena, P.; Nehra, S. Phytoextract mediated ZnO/MgO nanocomposites for photocatalytic and antibacterial activities. J. Photochem. Photobiol. A Chem. 2019, 385, 112049. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Ali, M.U.; Ali, A.; Shabbir, H.; Bin Nazeer, U.; Saleem, M.S. Solar light triggered catalytic performance of graphene-CuO nanocomposite for waste water treatment. Ceram. Int. 2017, 43, 10654–10660. [Google Scholar] [CrossRef]
- Mageshwari, K.; Nataraj, D.; Pal, T.; Sathyamoorthy, R.; Park, J. Improved photocatalytic activity of ZnO coupled CuO nanocomposites synthesized by reflux condensation method. J. Alloy. Compd. 2015, 625, 362–370. [Google Scholar] [CrossRef]
- Arunadevi, R.; Kavitha, B.; Rajarajan, M.; Suganthi, A.; Jeyamurugan, A. Investigation of the drastic improvement of photocatalytic degradation of Congo red by monoclinic Cd, Ba-CuO nanoparticles and its antimicrobial activities. Surf. Interfaces 2018, 10, 32–44. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, F.; Goei, R.; Zhou, Y. Facile fabrication of RGO-WO3 composites for effective visible light photocatalytic degradation of sulfamethoxazole. Appl. Catal. B Environ. 2017, 207, 93–102. [Google Scholar] [CrossRef]
- Cao, Q.W.; Zheng, Y.F.; Song, X.C. Enhanced visible-light-driven photocatalytic degradation of RhB by AgIO3/WO3 composites. J. Taiwan Inst. Chem. Eng. 2017, 70, 359–365. [Google Scholar] [CrossRef]
- Hakimi, M.; Morvaridi, M.; Hosseini, H.A.; Alimard, P. Preparation, characterization, and photocatalytic activity of Bi2O3–Al2O3 nanocomposite. Polyhedron 2019, 170, 523–529. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodriguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnol. 2008, 19, 145605. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R.; Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Vimercati, L.; Cavone, D.; Caputi, A.; De Maria, L.; Tria, M.; Prato, E.; Ferri, G.M. Nanoparticles: An experimental study of zinc nanoparticles toxicity on marine crustaceans. General overview on the health implications in humans. Front. Public Heal. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Monsé, C.; Hagemeyer, O.; Raulf, M.; Jettkant, B.; Van Kampen, V.; Kendzia, B.; Gering, V.; Kappert, G.; Weiss, T.; Ulrich, N.; et al. Concentration-dependent systemic response after inhalation of nano-sized zinc oxide particles in human volunteers. Part. Fibre Toxicol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Mojir Shaibani, P.M.; Prashanthi, K.; Sohrabi, A.; Thundat, T. Photocatalytic BiFeO3 nanofibrous mats for effective water treatment. J. Nanotechnol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Shen, J.; Li, N.; Ye, M. Hydrothermal preparation, characterization and enhanced properties of reduced graphene-BiFeO3 nanocomposite. Mater. Lett. 2013, 91, 42–44. [Google Scholar] [CrossRef]
- Roy, S.; Majumder, S. Recent advances in multiferroic thin films and composites. J. Alloy. Compd. 2012, 538, 153–159. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, C.; Wang, P.; Ng, D.H.L. Template-free hydrothermal synthesis of mesoporous MgO nanostructures and their applications in water treatment. Chem. Asian J. 2012, 7, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Casillas, J.E.; Campa-Molina, J.; Tzompantzi, F.; Arízaga, G.G.C.; López-Gaona, A.; Ulloa-Godínez, S.; Cano, M.E.; Barrera, A. Photocatalytic degradation of diclofenac using Al2O3-Nd2O3 binary oxides prepared by the sol-gel method. Materials 2020, 13, 1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Physica Status Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Shi, G.; Liu, J.; Chen, B.; Bao, Y.; Xu, J. Phase-controlled growth of cubic phase CuO nanoparticles by chemical vapor deposition. Physica Status Solidi A 2017, 214. [Google Scholar] [CrossRef]
- Sathyamoorthy, R.; Mageshwari, K.; Mali, S.S.; Priyadharshini, S.; Patil, P.S. Effect of organic capping agent on the photocatalytic activity of MgO nanoflakes obtained by thermal decomposition route. Ceram. Int. 2013, 39, 323–330. [Google Scholar] [CrossRef]
- Camacho, L.M.; Torres, A.; Saha, D.; Deng, S. Adsorption equilibrium and kinetics of fluoride on sol–gel-derived activated alumina adsorbents. J. Colloid Interface Sci. 2010, 349, 307–313. [Google Scholar] [CrossRef]
- Gaudet, J.R.; De La Riva, A.; Peterson, E.J.; Bolin, T.; Datye, A.K. Improved low-temperature CO oxidation performance of Pd supported on La-stabilized alumina. ACS Catal. 2013, 3, 846–855. [Google Scholar] [CrossRef]
- Ealet, B.; Elyakhloufi, M.; Gillet, E.; Ricci, M. Electronic and crystallographic structure of γ-alumina thin films. Thin Solid Films 1994, 250, 92–100. [Google Scholar] [CrossRef]
- Weng, B.; Wu, J.; Zhang, N.; Xu, Y.-J. Observing the role of graphene in boosting the two-electron reduction of oxygen in graphene-WO3 nanorod photocatalysts. Langmuir 2014, 30, 5574–5584. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.M. Structure and properties of WO3 thin films for electrochromic device application. J. Non Oxide Glasses 2013, 5, 1–8. [Google Scholar]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Hu, C.; Lu, T.; Chen, F.; Zhang, R. A brief review of graphene–metal oxide composites synthesis and applications in photocatalysis. J. Chin. Adv. Mater. Soc. 2013, 1, 21–39. [Google Scholar] [CrossRef]
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Jung, W. RGO-TiO2-ZnO composites: Synthesis, characterization, and application to photocatalysis. Appl. Catal. A Gen. 2015, 491, 52–57. [Google Scholar]
- Li, N.; Zheng, M.; Lu, H.; Hu, Z.; Shen, C.; Chang, X.; Ji, G.; Cao, J.; Shi, Y. High-rate lithium–sulfur batteries promoted by reduced graphene oxide coating. Chem. Commun. 2012, 48, 4106–4108. [Google Scholar] [CrossRef] [PubMed]
- Firmiano, E.G.S.; Cordeiro, M.A.L.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Leite, E.R. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst. Chem. Commun. 2012, 48, 7687–7689. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Pant, B.; Kim, H.J.; Amarjargal, A.; Park, C.H.; Tijing, L.D.; Kim, E.K.; Kim, C.S. A green and facile one-pot synthesis of Ag-ZnO/RGO nanocomposite with effective photocatalytic activity for removal of organic pollutants. Ceram. Int. 2013, 39, 5083–5091. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Wu, D.; Su, X.; Guo, W. Enhanced photocatalytic degradation of methylene blue over hexagonal WO3/graphene under visible-light irradiation. Kinet. Catal. 2017, 58, 710–719. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Cheng, G.; Guo, H.; Shen, Z.; Yang, L.; Zhao, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Preparing a photocatalytic Fe doped TiO2/rGO for enhanced bisphenol A and its analogues degradation in water sample. Appl. Surf. Sci. 2020, 505, 144640. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Sun, J.; Yu, C.; Li, Y.; Sun, J. Facile synthesis of novel ZnO/RGO hybrid nanocomposites with enhanced catalytic performance for visible-light-driven photodegradation of metronidazole. Mater. Chem. Phys. 2014, 145, 357–365. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Bahadur, S.A.; Swaminathan, M. Visible active reduced graphene oxide-BiVO4-ZnO ternary photocatalyst for efficient removal of ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
- Li, K.; Chen, T.; Yan, L.; Dai, Y.; Huang, Z.; Xiong, J.; Song, D.; Lv, Y.; Zeng, Z. Design of graphene and silica co-doped titania composites with ordered mesostructure and their simulated sunlight photocatalytic performance towards atrazine degradation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 90–99. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Huang, L.; Liu, X.; Han, Y.; Wang, L. La2Zr2O7/rGO synthesized by one-step sol-gel method for photocatalytic degradation of tetracycline under visible-light. Chem. Eng. J. 2020, 384, 123380. [Google Scholar] [CrossRef]
- Prabhakarrao, N.; Chandra, M.R.; Rao, T.S. Synthesis of Zr doped TiO2/reduced Graphene Oxide (rGO) nanocomposite material for efficient photocatalytic degradation of eosin blue dye under visible light irradiation. J. Alloy. Compd. 2017, 694, 596–606. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Sono-synthesis of nanocrystallized BiFeO3/reduced graphene oxide composites for visible photocatalytic degradation improvement of bisphenol A. Chem. Eng. J. 2016, 306, 204–213. [Google Scholar] [CrossRef]
- Peng, Y.; Ji, J.; Chen, D. Ultrasound assisted synthesis of ZnO/reduced graphene oxide composites with enhanced photocatalytic activity and anti-photocorrosion. Appl. Surf. Sci. 2015, 356, 762–768. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; AshokKumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochemistry 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Labhane, P.; Patle, L.; Huse, V.; Sonawane, G. Synthesis of reduced graphene oxide sheets decorated by zinc oxide nanoparticles: Crystallographic, optical, morphological and photocatalytic study. Chem. Phys. Lett. 2016, 661, 13–19. [Google Scholar] [CrossRef]
- Moitra, D.; Chandel, M.; Ghosh, B.K.; Jani, R.K.; Patra, M.K.; Vadera, S.R.; Ghosh, N.N. A simple ‘in situ’ co-precipitation method for the preparation of multifunctional CoFe2O4-reduced graphene oxide nanocomposites: Excellent microwave absorber and highly efficient magnetically separable recyclable photocatalyst for dye degradation. RSC Adv. 2016, 6, 76759–76772. [Google Scholar] [CrossRef]
- Rahmat, S.T.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Facile fabrication of rGO/Rutile TiO2 nanowires as photocatalyst for Cr(VI) Reduction. Mater. Today Proc. 2019, 17, 1143–1151. [Google Scholar] [CrossRef]
- Kumar, G.; Nikolla, E.; Linic, S.; Medlin, J.W.; Janik, M.J. Multicomponent catalysts: Limitations and prospects. ACS Catal. 2018, 8, 3202–3208. [Google Scholar] [CrossRef]
- Noormohammadi, E.; Sanjabi, S. Photocatalytic activity and wettability of rGO/TiO2 nanocomposites prepared by electrophoretic co-deposition. Surf. Rev. Lett. 2019, 27, 9–11. [Google Scholar] [CrossRef]
- Ramos, P.G.; Luyo, C.; Sánchez, L.A.; Gomez, E.D.; Rodriguez, J.M. The spinning voltage influence on the growth of ZnO-rGO nanorods for photocatalytic degradation of methyl orange dye. Catalysts 2020, 10, 660. [Google Scholar] [CrossRef]
- Park, J.A.; Yang, B.; Lee, J.; Kim, I.G.; Kim, J.-H.; Choi, J.-W.; Park, H.-D.; Nah, I.W.; Lee, S.-H. Ultrasonic spray pyrolysis synthesis of reduced graphene oxide/anatase TiO2 composite and its application in the photocatalytic degradation of methylene blue in water. Chemosphere 2018, 191, 738–746. [Google Scholar] [CrossRef]
- Prabhu, S.; Pudukudy, M.; Sohila, S.; Harish, S.; Navaneethan, M.; Ramesh, R.; Hayakawa, Y. Synthesis, structural and optical properties of ZnO spindle/reduced graphene oxide composites with enhanced photocatalytic activity under visible light irradiation. Opt. Mater. 2018, 79, 186–195. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.-K. Integrated ternary nanocomposite of TiO2/NiO/reduced graphene oxide as a visible light photocatalyst for efficient degradation of o-chlorophenol. J. Environ. Manag. 2016, 181, 563–573. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Küppers, S. Calcined layered double hydroxides/reduced graphene oxide composites with improved photocatalytic degradation of paracetamol and efficient oxidation-adsorption of As(III). Appl. Catal. B Environ. 2018, 225, 550–562. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, N.; Chauhan, R.; Singh, V.; Srivastava, V.C.; Bhatnagar, R. Growth of hierarchical ZnO nano flower on large functionalized rGO sheet for superior photocatalytic mineralization of antibiotic. Chem. Eng. J. 2020, 392, 123746. [Google Scholar] [CrossRef]
- Ranjith, R.; Renganathan, V.; Chen, S.-M.; Selvan, N.S.; Rajam, P.S. Green synthesis of reduced graphene oxide supported TiO2/Co3O4 nanocomposite for photocatalytic degradation of methylene blue and crystal violet. Ceram. Int. 2019, 45, 12926–12933. [Google Scholar] [CrossRef]
- Li, A.D.; Liu, W.C. Optical properties of ferroelectric nanocrystal/polymer composites. Phys. Prop. Appl. Polym. Nanocompos. Published online. 2010, 108–158. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, H.; Zhang, Y.C.; He, W.L.; Zhu, M.K.; Wang, B.; Yan, H. Hydrothermal synthesis of zinc oxide powders with controllable morphology. Ceramic Int. 2011, 1, 93–97. [Google Scholar] [CrossRef]
- O’Hare, D. Hydrothermal synthesis. Encycl. Mater. Sci. Technol. Published online. 2001, 3989–3992. [Google Scholar] [CrossRef]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal synthesis of rGO-TiO2 composites as high-performance UV photocatalysts for ethylparaben degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 133, 452–459. [Google Scholar] [CrossRef]
- Lv, K.; Fang, S.; Si, L.; Xia, Y.; Ho, W.; Li, M. Fabrication of TiO2 nanorod assembly grafted rGO (rGO@TiO2-NR) hybridized flake-like photocatalyst. Appl. Surf. Sci. 2017, 391, 218–227. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Zhang, Y.; Ma, Y.; Zhang, Q.; Pu, H.; Xu, W.; Gao, D.; Wang, B.; Qi, X. Preparation of RGO/TiO2 photocatalyst and the mechanism of its hydrothermal process. J. Chin. Chem. Soc. 2019, 66, 734–739. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, Á.; Manassero, A.; Satuf, M.L.; Alfano, O.M.; Casas, J.A.; Bahamonde, A. TiO2-rGO photocatalytic degradation of an emerging pollutant: Kinetic modelling and determination of intrinsic kinetic parameters. J. Environ. Chem. Eng. 2019, 7, 103406. [Google Scholar] [CrossRef]
- Romeiro, F.C.; Rodrigues, M.A.; Silva, L.A.; Catto, A.C.; da Silva, L.F.; Longo, E.; Nossol, E.; Lima, R.C. rGO-ZnO nanocomposites for high electrocatalytic effect on water oxidation obtained by microwave-hydrothermal method. Appl. Surf. Sci. 2017, 423, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Shang, E.; Li, Y.; Niu, J.; Li, S.; Zhang, G.; Wang, X. Photocatalytic degradation of perfluorooctanoic acid over Pb-BiFeO3/rGO catalyst: Kinetics and mechanism. Chemosphere 2018, 211, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Khamboonrueang, D.; Srirattanapibul, S.; Tang, I.-M.; Thongmee, S. TiO2∙rGO nanocomposite as a photo catalyst for the reduction of Cr6+. Mater. Res. Bull. 2018, 107, 236–241. [Google Scholar] [CrossRef]
- Srirattanapibul, S.; Tang, I.-M.; Thongmee, S. Photo catalytic reduction of Cr6+ by ZnO decorated on reduced graphene oxide (rGO) Nanocomposites. Mater. Res. Bull. 2020, 122. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Yang, J.; Cai, X.; Fu, L. One-pot synthesis of cuprous oxide-reduced graphene oxide nanocomposite with enhanced photocatalytic and electrocatalytic performance. Physica E Low Dimens. Syst. Nanostructures 2016, 77, 122–126. [Google Scholar] [CrossRef]
- Zhigang, N. Reduced graphene oxide-cuprous oxide hybrid nanopowders: Hydrothermal synthesis and enhanced photocatalytic performance under visible light irradiation. Mater. Sci. Semicond. Process. 2014, 23, 78–84. [Google Scholar] [CrossRef]
- Sun, L.; Wang, G.; Hao, R.; Han, D.; Cao, S. Solvothermal fabrication and enhanced visible light photocatalytic activity of Cu2O-reduced graphene oxide composite microspheres for photodegradation of Rhodamine, B. Appl. Surf. Sci. 2015, 358, 91–99. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, L.; Liu, L.; Wang, X.; Sun, J.; Wu, S.; Deng, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the Cu2O–reduced graphene oxide interface to enhance photocatalytic degradation of organic pollutants under visible light. Appl. Catal. B Environ. 2016, 181, 495–503. [Google Scholar] [CrossRef]
- Cauqui, M.A.; Rodriguez-Izquierdo, J.M. Application of the sol-gel methods to catalyst preparation. J. Non Cryst. Solids 1992, 92, 724–738. [Google Scholar] [CrossRef]
- Banerjee, S.; Benjwal, P.; Singh, M.; Kar, K.K. Graphene oxide (rGO)-metal oxide (TiO2/Fe3O4) based nanocomposites for the removal of methylene blue. Appl. Surf. Sci. 2018, 439, 560–568. [Google Scholar] [CrossRef]
- Alizadeh, S.; Fallah, N.; Nikazar, M. An ultrasonic method for the synthesis, control and optimization of CdS/TiO2 core–shell nanocomposites. RSC Adv. 2019, 9, 4314–4324. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.P.; Kale, D.P.; Kar, S.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K.; Sonawane, S.H. Ultrasound assisted preparation of rGO/TiO2 nanocomposite for effective photocatalytic degradation of methylene blue under sunlight. Nano Struct. Nano Objects 2020, 21, 100407. [Google Scholar] [CrossRef]
- Deraz, N.M. The comparative jurisprudence of catalysts preparation methods: I. precipitation and impregnati. J. Ind. Environ. Chem. 2018, 2, 19–21. [Google Scholar]
- Sietsma, J.R.; Van Dillen, A.J.; De Jongh, P.E.; De Jong, K.P. Application of ordered mesoporous materials as model supports to study catalyst preparation by impregnation and drying. Stud. Surf. Sci. Catal. 2006, 162, 95–102. [Google Scholar]
- Haber, J.; Block, J.H.; Delmon, B. Manual of methods and procedures for catalyst characterization. Pure Appl. Chem. 1995, 67, 1257–1306. [Google Scholar] [CrossRef] [Green Version]
- Babu, S.G.; Vinoth, R.; Neppolian, B.; Dionysiou, D.D.; AshokKumar, M. Diffused sunlight driven highly synergistic pathway for complete mineralization of organic contaminants using reduced graphene oxide supported photocatalyst. J. Hazard. Mater. 2015, 291, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, M.; Di Paola, A.; Yurdakal, S.; Palmisano, L. Chapter 2—Preparation of Catalysts and Photocatalysts Used for Similar Processes. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Springer: Berlin, Germany, 2019; pp. 25–56. [Google Scholar] [CrossRef]
- Pouya, A.; Jerzy, S.; Krasinski, R.V.; Lobat, T.; Vashaee, D. Electrophoretic Deposition (EPD): 17 Fundamentals and Applications from Nano- to Microscale Structures. In Handbook of Nanoelectrochemistry; Aliofkhazraei, M., Hamdy Makhlouf, A.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Reddy, A.B.; Reddy, G.S.M.; Sivanjineyulu, V.; Jayaramudu, J.; Varaprasad, K.; Sadiku, E.R. Hydrophobic/Hydrophilic Nanostructured Polymer Blends. In Micro and Nano Technologies, Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 385–411. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.-C.; Jayanthi, G.V. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Tsai, S.C.; Song, Y.L.; Tsai, C.S.; Chiu, W.Y.; Lin, H.M. Ultrasonic spray pyrolysis for nanoparticles synthesis. Mater Sci. 2004, 39, 3647–3657. [Google Scholar] [CrossRef]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Rahimi, K.; Zafarkish, H.; Yazdani, A. Reduced graphene oxide can activate the sunlight-induced photocatalytic effect of NiO nanowires. Mater. Des. 2018, 144, 214–221. [Google Scholar] [CrossRef]
- Suresh, R.; Mangalaraja, R.V.; Mansilla, H.D.; Yáñez, J. Reduced Graphene Oxide-Based Photocatalysis. In Green Photocatalyst; Naushad, M., Rajendran, S., Lichtfouse, E., Eds.; Springer: Chemnitz, Germany, 2020; Volume 34, pp. 145–166. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, X.; Liu, W.; Tan, P.; Chen, H.; Wu, L.; Ma, C.; Xiong, X.; Pan, J. Photocorrosion inhibition and high-efficiency photoactivity of porous g-C3N4/Ag2CrO4 composites by simple microemulsion-assisted co-precipitation method. Appl. Catal. B Environ. 2017, 204, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Khavar, A.H.C.; Moussavi, G.; Mahjoub, A.R.; Satari, M.; Abdolmaleki, P. Synthesis and visible-light photocatalytic activity of In,S-TiO2@rGO nanocomposite for degradation and detoxification of pesticide atrazine in water. Chem. Eng. J. 2018, 345, 300–311. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Shen, L.; Li, R.; Tan, C.; Chen, T.; Liu, H.; Liu, Y.; Cai, Z.; Liu, G.; et al. Insights into the synergetic mechanism of a combined vis-RGO/TiO2/peroxodisulfate system for the degradation of PPCPs: Kinetics, environmental factors and products. Chemosphere 2019, 216, 341–351. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Ma, F.; Xu, Y. Synergistic effects and kinetics of rGO-modified TiO2 nanocomposite on adsorption and photocatalytic degradation of humic acid. J. Environ. Manag. 2019, 235, 293–302. [Google Scholar] [CrossRef]






| Pollutants. | Catalyst | Method | % Removal | Band Gap (eV) | Time of Reaction (min) | Sources of Light | Ref. |
|---|---|---|---|---|---|---|---|
| Phenol, Methyl orange (MO) Rhodamine B (RhB) | TiO2 | Hydrolysis deposition and calcination crystallization | Phenol: 54, MO: 80.7, RhB: 84.2 | 3.05 | Phenol: 360, Rh B and MO: 45 | UV lamp | [38] |
| Tetracycline hydrochloride | TiO2 | Sol-gel (450 ℃ for 2 h) | 18.2 | 3.17 | 120 | 30 W LED lamp | [39] |
| Ponceau BS | TiO2 | Sol-gel (450 ℃ for 4 h) | 24.45 | 3.22 | 27 | 125 W mercury lamp | [40] |
| Methyl orange | TiO2 | Impregnation | 42.98 | - | 60 | 150 W mercury lamp | [41] |
| Rh B | TiO2 | Solvothermal (180 ℃ for 24 h) | 34.1 | 3.06 | 150 | 300 W Xe arc lamp | [42] |
| Methyl orange | TiO2 | Hydrothermal (180 ℃ for 18 h) and calcination (350℃ for 2 h) | 4.6 | 3.10 | 60 | 500 W Xe lamp | [43] |
| Methyl orange | TiO2 | Polyol (500 ℃ for 2 h) | 24 | - | 90 | 12 units of 100 W fluorescent lamps | [44] |
| Congo red | ZnO | Microwave-assisted hydrothermal | 53.1 | 3.25 | 60 | 100 W UV lamp | [45] |
| Methyl orange | ZnO | Bio synthesis (450 ℃) | 83.99 | 3.32 | 120 | 8 W mercury lamp | [46] |
| Flumequine | ZnO | Chemical precipitation | 11 | 3.24 | 240 | UV lamp | [47] |
| Methylene blue | ZnO | Seed layer deposition and chemical bath deposition | 54 | 3.32 | 240 | Solar light | [48] |
| Phenol | ZnO | In situ preparation (hydrolysis) | 56 | - | 240 | 40 W UVC lamp | [49] |
| Doxorubicin | BiFeO3 | Thermal decomposition (480 ℃ for 1 h) | 79 | 2.1 | 180 | UV lamp | [50] |
| Tetracycline | BiFeO3 | Sol-gel (550 ℃ for 2 h) | 27.3 | 2.09 | 180 | 300 W Xe lamp | [51] |
| Methyl violet | Bi2Fe4O9 | Hydrothermal (180 ℃ for 72 h) | 10.6 | - | 180 | Visible light | [52] |
| Rhodamine B | Bi25FeO40 | Hydrothermal (180 ℃) | 62 | 1.8 | 240 | 500 W Mercury-Xenon lamps | [53] |
| Rhodamine B | Bi25FeO40 | Hydrothermal (150 ℃ for 2 h) | 28 | 2.78 | 180 | 240 W Xe lamp | [54] |
| Rhodamine B, BPA | BiFeO3 | Co-precipitation | Rh B: 10, BPA: 7 | 2.16 | Rh B: 60, BPA: 120 | 250 W Tungsten lamp (visible) | [55] |
| Methyl orange | MgO | Sonication assisted solvothermal (180 ℃ for 10 h) | 43 | - | 120 | 90 W UVC lamp | [56] |
| Methylene blue | MgO | Biosynthesis (Ricinus communis l.) | 49.9 | 3.54 | 120 | Solar light | [57] |
| Methylene blue | CuO | Thermal decomposition (350 ℃ for 2 h) | 75 | 1.66 | 80 | Solar light | [58] |
| Methyl orange, Methylene blue | CuO | Reflux condensation | MB: 93.66, MO: 95.63 | 1.29 | MO: 120, MB: 180 | UV lamp | [59] |
| Congo red | CuO | Co-precipitation | 77 | 1.60 | 180 | 300 W Xe lamp | [60] |
| Sulfamethoxazole | WO3 | Hydrothermal (140 ℃ for 8 h) | 46.2 | 2.75 | 180 | 200 W Xe lamp | [61] |
| Rhodamine B | WO3 | Hydrothermal (180 ℃ for 12 h) | 14.7 | 2.46 | 140 | 300 W Xe lamp | [62] |
| Methylene blue | Al2O3 | Sol-gel (600 ℃ for 8 h) | 14 | 4.9 | 90 | 11 W UV lamp | [63] |
| Pollutants. | Catalyst | Method | Optimum Concentration of rGO/GO | % Removal | Band Gap (eV) | Time of Reaction (min) | Sources of Light | Ref. |
|---|---|---|---|---|---|---|---|---|
| Methylene Blue | BiFeO3/ rGO | Hydrothermal (160 ℃ for 6 h) | 3 mg/mL | 92 | 2.4 | 140 | 150 W Xe lamp | [26] |
| Chromium (VI) | rGO-TiO2-ZnO | Hydrothermal (180 ℃ for 20 h) | 0.5 mg/mL | 63 | - | 120 | 100 W Mercury lamp | [87] |
| Methylene blue, Reactive black 5 | Ag-ZnO/ rGO | Hydrothermal (140 ℃ for 2 h) | 10 mg | MB: 61, RB5: 85 | - | 200 | Mercury-vapor lamp | [91] |
| Tetracycline, Chlortetracycline, Oxytetracycline, Doxycycline | BiVO4/ TiO2/ rGO | Hydrothermal (200 ℃ for 6 h) | 0.5% | TC: 96, OTC: 98, CTC: 96, DC: 100 | 2.29 | 120 | 1000 W Xenon lamp | [92] |
| Methylene blue | WO3/rGO | Hydrothermal (120 ℃ for 24 h) | 0.2% | 98 | 2.7 | 360 | 300 W Xe lamp | [93] |
| Tetracycline | La2Zr2O7/ rGO | Sol gel | 0.5 g | 82.1 | 2.42 | 40 | 300 W Xe lamp | [98] |
| Eosin blue | Zr-TiO2/ rGO | Sol-gel (70 ℃ for 24 h) | 5% | 96 | 2.95 | 90 | 400 W mercury vapour lamp | [99] |
| Bisphenol A (BPA) | BiFeO3/rGO | Ultrasonic (40 kHz, 10 min, 40 °C) | 4% | 98 | 1.9–2.0 | 70 | 55 W Fluorescent lamps | [100] |
| Methylene blue, Acid red 249 | ZnO/rGO | Ultrasonic (40 kHz, 1 h, 500 W) | 2% | MB: 92.9, AR249: 94.8 | - | 120 | 15 W UV lamp | [101] |
| Methyl orange | Cu-TiO2/ rGO | Wet impregnation | 2% | 99 | 3.08 | 90 | UV lamp | [102] |
| Methylene blue | ZnO/rGO | Wet impregnation | 0.5 g | 94 | 3.17 | 45 | 125 W UV lamp | [103] |
| Bisphenol A (BPA) | Bi2Fe4O9/ rGO | Co-precipitation (95 °C) | 4.5% | 80 | 2.3 | 180 | 150 W Xe arc lamp | [22] |
| Methyl orange, Rhodamine B, Methylene blue | CoFe2O4/ rGO | Co-precipitation (160 ℃) | 25% | All dyes: 100 | 0.85 | MO: 60, MB: 75, Rh B: 45 | 100 W reading lamp | [104] |
| Chromium (VI) | rGO/TiO2 | Electrophoretic | 0.1 mg/mL | 100 | - | 60 | Solar simulator | [105] |
| Methylene blue | rGO/TiO2 | Electrophoretic | 0.8 g/L | 19 | - | 30 | UV light | [107] |
| Methyl orange | ZnO/rGO | Electrospinning | 0.2% | 99 | - | 360 | 220 W UVA lamp | [108] |
| Methylene blue | rGO/TiO2 | Ultrasonic spray pyrolysis | 5% | UVA: 40.8 UVC: 100 | 2.72 | UVA: 15 UVC: 60 | UVA or UVC lamp | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Kaus, N.H.; Rithwan, A.F.; Adnan, R.; Ibrahim, M.L.; Thongmee, S.; Mohd Yusoff, S.F. Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation. Catalysts 2021, 11, 302. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030302
Mohd Kaus NH, Rithwan AF, Adnan R, Ibrahim ML, Thongmee S, Mohd Yusoff SF. Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation. Catalysts. 2021; 11(3):302. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030302
Chicago/Turabian StyleMohd Kaus, Noor Haida, Ahmad Fadhil Rithwan, Rohana Adnan, Mohd Lokman Ibrahim, Sirikanjana Thongmee, and Siti Fairus Mohd Yusoff. 2021. "Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation" Catalysts 11, no. 3: 302. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030302