Insight into the Promoting Role of Er Modification on SO2 Resistance for NH3-SCR at Low Temperature over FeMn/TiO2 Catalysts
Abstract
:1. Introduction
2. Results
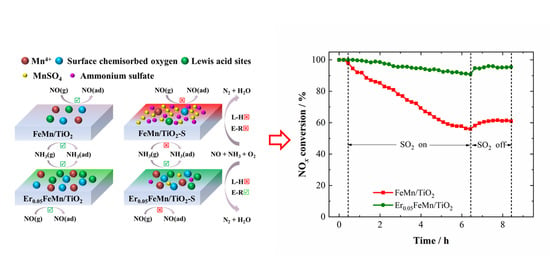
2.1. Low Temperature SCR Activity and SO2 Resistance
2.2. BET Results
2.3. XRD Analysis
2.4. H2-TPR Analysis
2.5. XPS Analysis
2.6. TG Analysis
2.7. In-Situ DRIFTS
2.7.1. Adsorption of SO2
2.7.2. Effect of SO2 on NH3 Adsorption on Catalyst Surface
2.7.3. Effect of SO2 on NO + O2 Adsorption on Catalyst Surface
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalyst Activity Test
4.3. Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, Z.H.; Ren, S.; Chen, Z.C.; Yang, J.; Zhou, Y.H.; Jiang, L.J.; Yang, C. Deactivation effect of CaO on Mn-Ce/AC catalyst for SCR of NO with NH3 at low temperature. Catalysts 2020, 10, 873. [Google Scholar] [CrossRef]
- Zhu, N.; Shan, W.P.; Lian, Z.H.; Zhang, Y.; Liu, K.; He, H. A superior Fe-V-Ti catalyst with high activity and SO2 resistance for the selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 382, 120970. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.D.; Xu, L.; Ohnishi, T.; Yanaba, Y.; Ogura, M.; Wakihara, T.; Okubo, T. Understanding the high hydrothermal stability and NH3-SCR activity of the fast-synthesized ERI zeolite. J. Catal. 2020, 391, 346–356. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Maitarad, P.; Wang, P.L.; Han, L.P.; Yan, T.T.; Li, H.R.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Alkali-resistant NOx reduction over SCR catalysts via boosting NH3 adsorption rates by in situ constructing the sacrificed sites. Environ. Sci. Technol. 2020, 54, 13314–13321. [Google Scholar] [CrossRef]
- Ye, D.; Wang, X.X.; Liu, H.; Wang, H.N. Insights into the effects of sulfate species on CuO/TiO2 catalysts for NH3-SCR reactions. Mol. Catal. 2020, 496, 111191. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ma, S.B.; Li, Z.B.; Yuan, F.L.; Niu, X.Y.; Zhu, Y.J. Synthesis of CenTiOx flakes with hierarchical structure and its enhanced activity for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2020, 392, 123801. [Google Scholar] [CrossRef]
- Zhang, N.Q.; Li, L.C.; Guo, Y.Z.; He, J.D.; Wu, R.; Song, L.Y.; Zhang, G.Z.; Zhao, J.S.; Wang, D.S.; He, H. A MnO2-based catalyst with H2O resistance for NH3-SCR: Study of catalytic activity and reactants-H2O competitive adsorption. Appl. Catal. B Environ. 2020, 270, 118860. [Google Scholar] [CrossRef]
- Fan, Y.M.; Ling, W.; Huang, B.C.; Dong, L.F.; Yu, C.L.; Xi, H.X. The synergistic effects of cerium presence in the framework and the surface resistance to SO2 and H2O in NH3-SCR. J. Ind. Eng. Chem. 2017, 56, 108–119. [Google Scholar] [CrossRef]
- Ye, L.M.; Lu, P.; Chen, X.B.; Fang, P.; Peng, Y.; Li, J.H.; Huang, H.B. The deactivation mechanism of toluene on MnOx-CeO2 SCR catalyst. Appl. Catal. B Environ. 2020, 277, 119257. [Google Scholar] [CrossRef]
- Yan, T.; Liu, Q.; Wang, S.H.; Xu, G.; Wu, M.H.; Chen, J.J.; Li, J.H. Promoter rather than inhibitor: Phosphorus incorporation accelerates the activity of V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. ACS Catal. 2020, 10, 2747–2753. [Google Scholar] [CrossRef]
- Yan, R.; Lin, S.X.; Li, Y.L.; Liu, W.M.; Mi, Y.Y.; Tang, C.J.; Wang, L.; Wu, P.; Peng, H.G. Novel shielding and synergy effects of Mn-Ce oxides confined in mesoporous zeolite for low temperature selective catalytic reduction of NOx with enhanced SO2/H2O tolerance. J. Hazard. Mater. 2020, 396, 122592. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Guo, R.T.; Shi, X.; Liu, X.Y.; Qin, H.; Liu, Y.Z.; Duan, C.P.; Guo, D.Y.; Pan, W.G. The superior performance of CoMnOx catalyst with ball-flowerlike structure for low-temperature selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2020, 381, 122753. [Google Scholar] [CrossRef]
- Lyu, Z.K.; Niu, S.L.; Lu, C.M.; Zhao, G.J.; Gong, Z.Q.; Zhu, Y. A density functional theory study on the selective catalytic reduction of NO by NH3 reactivity of α-Fe2O3 (001) catalyst doped by Mn, Ti, Cr and Ni. Fuel 2020, 267, 117147. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.Q.; Ma, S.B.; Yuan, F.L.; Li, Z.B.; Zhu, Y.J. Excellent selective catalytic reduction of NOx by NH3 over Cu/SAPO-34 with hierarchical pore structure. Chem. Eng. J. 2020, 379, 122376. [Google Scholar] [CrossRef]
- Zhao, J.J.; Yu, Y.B.; Han, X.; He, H. Fuel reforming over Ni-based catalysts coupled with selective catalytic reduction of NOx. Chin. J. Catal. 2013, 34, 1407–1417. [Google Scholar] [CrossRef]
- Du, H.; Han, Z.T.; Wang, Q.M.; Gao, Y.; Gao, C.; Dong, J.M.; Pan, X.X. Effects of ferric and manganese precursors on catalytic activity of Fe-Mn/TiO2 catalysts for selective reduction of NO with ammonia at low temperature. Environ. Sci. Pollut. Res. 2020, 27, 40870–40881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT study of the SO2 effect on low-temperature SCR reaction over Fe-Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Deng, B.Y.; Zhang, Z.Q.; Wu, Z.B.; Tang, X.J.; Yao, S.L.; Lu, H. Effect of Zr addition on the low-temperature SCR activity and SO2 tolerance of Fe-Mn/Ti catalysts. J. Phys. Chem. C 2012, 118, 14866–14875. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, Y.E.; Kumar, P.A.; Lee, K.Y.; Ha, H.P. Sb modified Fe-Mn/TiO2 catalyst for the reduction of NOx with NH3 at low temperatures. Res. Chem. Intermed. 2018, 44, 3737–3751. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Zhang, Y.P.; Xiao, R.; Huang, T.J.; Shen, K. Novel holmium-modified Fe-Mn/TiO2 catalysts with a broad temperature window and high sulfur dioxide tolerance for low-temperature SCR. Catal. Commun. 2017, 88, 64–67. [Google Scholar] [CrossRef]
- Hou, X.X.; Chen, H.P.; Liang, Y.H.; Yang, X.; Wei, Y.L. Pr-doped modified Fe-Mn/TiO2 catalysts with a high activity and SO2 tolerance for NH3-SCR at low-temperature. Catal. Lett. 2020, 150, 1041–1048. [Google Scholar] [CrossRef]
- Hou, X.X.; Chen, H.P.; Liang, Y.H.; Wei, Y.L.; Li, Z.Q. La modified Fe-Mn/TiO2 catalysts to improve SO2 resistance for NH3-SCR at low-temperature. Catal. Surv. Asia 2020, 24, 291–299. [Google Scholar] [CrossRef]
- Yang, P.; Lu, C.; Hua, N.P.; Du, Y.K. Titanium dioxide nanoparticles Co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater. Lett. 2002, 57, 794–801. [Google Scholar] [CrossRef]
- Mao, C.Y.; Li, W.J.; Wu, F.; Dou, Y.Y.; Fang, L.; Ruan, H.B.; Kong, C.Y. Effect of Er doping on microstructure and optical properties of ZnO thin films prepared by sol-gel method. J. Mater. Sci.-Mater. Electron. 2015, 26, 8732–8739. [Google Scholar] [CrossRef]
- Gao, J.Q.; Jiang, R.Z.; Wang, J.; Wang, B.X.; Li, K.; Kang, P.L.; Li, Y.; Zhang, X.D. Sonocatalytic performance of Er3+:YAlO3/TiO2-Fe2O3 in organic dye degradation. Chem. Eng. J. 2011, 168, 1041–1048. [Google Scholar] [CrossRef]
- Vargas, M.A.L.; Casanova, M.; Trovarelli, A.; Busca, G. An ir study of thermally stable V2O5-WO3-TiO2 SCR catalysts modified with silica and rare-earths (Ce, Tb, Er). Appl. Catal. B Environ. 2007, 75, 303–311. [Google Scholar] [CrossRef]
- Casanova, M.; Llorca, J.; Sagar, A.; Schermanz, K.; Trovarelli, A. Mixed iron-erbium vanadate NH3-SCR catalysts. Catal. Today 2015, 241, 159–168. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kwon, D.W.; Ha, H.P. Er composition (X)-mediated catalytic properties of Ce1-xErxVO4 surfaces for selective catalytic NOx reduction with NH3 at elevated temperatures. Catal. Today 2021, 359, 65–75. [Google Scholar] [CrossRef]
- Jin, Q.J.; Shen, Y.S.; Zhu, S.M.; Li, X.H.; Hu, M. Promotional effects of Er incorporation in CeO2(ZrO2)/TiO2 for selective catalytic reduction of NO by NH3. Chin. J. Catal. 2016, 37, 1521–1529. [Google Scholar] [CrossRef]
- Mu, J.C.; Li, X.Y.; Sun, W.B.; Fan, S.Y.; Wang, X.Y.; Wang, L.; Qin, M.C.; Gan, G.Q.; Yin, Z.F.; Zhang, D.K. Enhancement of low-temperature catalytic activity over a highly dispersed Fe-Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Chou, Y.H.; Su, Z.H.; Yao, L.; Cao, J.; Jiang, L.J.; Hu, G.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3-SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Jiang, L.J.; Liu, Q.C.; Ran, G.L.; Kong, M.; Ren, S.; Yang, J.; Li, J.L. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.H.; Guang, J.Y.; Guo, D.W.; Wang, L.F.; Li, X.H. Ceria modified FeMnOx-enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2015, 166–167, 37–44. [Google Scholar] [CrossRef]
- Chang, H.Z.; Chen, X.Y.; Li, J.H.; Ma, L.; Wang, C.Z.; Liu, C.X.; Schwank, J.W.; Hao, J.M. Improvement of activity and SO2 tolerance of Sn-modified MnOx-CeO2 catalysts for NH3-SCR at low temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.R.; Wang, Y.; He, C.; Niu, C.M. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, G.B.; Wu, P.; Zhuang, K.; Shen, K.; Wang, S.; Huang, T.J. Effect of SO2 on the low-temperature denitrification performance of Ho-modified Mn/Ti catalyst. Chem. Eng. J. 2019, 400, 122597. [Google Scholar] [CrossRef]
- Gan, L.N.; Chen, J.J.; Peng, Y.; Yu, J.; Tran, T.; Li, K.Z.; Wang, D.; Xu, G.W.; Li, J.H. NOx removal over V2O5/WO3-TiO2 prepared by a grinding method: Influence of the precursor on vanadium dispersion. Ind. Eng. Chem. Res. 2018, 57, 150–157. [Google Scholar] [CrossRef]
- Chen, H.F.; Xia, Y.; Huang, H.; Gan, Y.P.; Tao, X.Y.; Liang, C.; Luo, J.M.; Fang, R.Y.; Zhang, J.; Zhang, W.K.; et al. Highly dispersed surface active species of Mn/Ce/TiW catalysts for high performance at low temperature NH3-SCR. Chem. Eng. J. 2017, 330, 1195–1202. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Z.X.; Guo, H.Q.; Li, K.; Zhang, Z.X.; Cui, M.S.; Yang, Y.P. Selective preparation of monocyclic aromatic hydrocarbons from ex-situ catalytic fast pyrolysis of pine over Ti(SO4)2-Mo2N/HZSM-5 catalyst. Fuel 2019, 243, 88–96. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Kapteijn, F.; Vanlangeveld, A.D.; Moulijn, J.A.; Andreini, A.; Vuurman, M.A.; Turek, A.M.; Jehng, J.M.; Wachs, I.E. Alumina-supported manganese oxide catalysts: I. Characterization: Effect of precursor and loading. J. Catal. 1994, 150, 94–104. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Li, R.M.; Wang, M.J.; Li, Y.S.; Tong, Y.M.; Yang, P.P.; Zhu, Y.J. Two steps synthesis of cetiox oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B Environ. 2021, 282, 119542. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.R.; Hu, H.; Hu, X.N.; Shi, L.Y.; Zhang, D.S. Promotional effects of zirconium doped CeVO4 for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2016, 183, 269–281. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.T.; Weng, X.L.; Wang, Y.; Wu, Z.B.; Wang, H.Q. DRIFT studies on the selectivity promotion mechanism of Ca-modified Ce-Mn/TiO2 catalysts for low-temperature NO reduction with NH3. J. Phys. Chem. C 2012, 116, 16582–16592. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, X.X.; Zhan, L.; Li, C.; Qiao, W.M.; Ling, L.C. Effect of SO2 on activated carbon honeycomb supported CeO2-MnOx catalyst for NO removal at low temperature. Ind. Eng. Chem. Res. 2015, 54, 2274–2278. [Google Scholar] [CrossRef]
- Romano, E.J.; Schulz, K.H. A XPS investigation of SO2 adsorption on ceria-zirconia mixed-metal oxides. Appl. Surf. Sci. 2005, 246, 262–270. [Google Scholar] [CrossRef]
- Sun, C.Z.; Liu, H.; Chen, W.; Chen, D.Z.; Yu, S.H.; Liu, A.N.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jin, R.B.; Wang, H.Q.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.T.; Li, S.H.; Zhang, W.; Du, X.Y.; Huang, L.; Zhu, Y.C.; Zhai, Y.B.; Zeng, G.M. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal. Chem. Eng. J. 2019, 371, 781–795. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jiang, B.Q.; Liu, Y.; Wang, H.Q.; Jin, R.B. DRIFT study of manganese/titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef]
- Zuo, J.L.; Chen, Z.H.; Wang, F.R.; Yu, Y.H.; Wang, L.F.; Li, X.H. Low-temperature selective catalytic reduction of NOx with NH3 over novel Mn-Zr mixed oxide catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Lian, Z.H.; Liu, F.D.; Shan, W.P.; He, H. Improvement of Nb doping on SO2 resistance of VOx/CeO2 catalyst for the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R.T.; Li, M.Y.; Sun, P.; Liu, S.M.; Pan, W.G.; Liu, S.W.; Sun, X. Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study. Fuel 2018, 223, 385–393. [Google Scholar] [CrossRef]
- Jin, R.B.; Liu, Y.; Wang, Y.; Cen, W.L.; Wu, Z.B.; Wang, H.Q.; Weng, X.L. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B Environ. 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Tong, Y.M.; Li, Y.S.; Li, Z.B.; Wang, P.Q.; Zhang, Z.P.; Zhao, X.Y.; Yuan, F.L.; Zhu, Y.J. Influence of sm on the low temperature NH3-SCR of NO activity and H2O/SO2 resistance over the SmaMnNi2Ti7Ox (a = 0.1, 0.2, 0.3, 0.4) catalysts. Appl. Catal. A-Gen. 2020, 590, 117333. [Google Scholar] [CrossRef]
- Zhang, B.L.; Liebau, M.; Suprun, W.; Liu, B.; Zhang, S.G.; Gläser, R. Suppression of N2O formation by H2O and SO2 in the selective catalytic reduction of NO with NH3 over a Mn/Ti-Si catalyst. Catal. Sci. Technol. 2019, 9, 4759–4770. [Google Scholar] [CrossRef]
- Xiong, S.C.; Peng, Y.; Wang, D.; Huang, N.; Zhang, Q.F.; Yang, S.J.; Chen, J.J.; Li, J.H. The role of the Cu dopant on a Mn3O4 spinel SCR catalyst: Improvement of low-temperature activity and sulfur resistance. Chem. Eng. J. 2020, 387, 124090. [Google Scholar] [CrossRef]
- Xie, S.Z.; Li, L.L.; Jin, L.J.; Wu, Y.H.; Liu, H.; Qin, Q.J.; Wei, X.L.; Liu, J.X.; Dong, L.H.; Li, B. Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for MH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl. Surf. Sci. 2020, 515, 146014. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Han, Z.; Wu, X.; Li, C.; Gao, Y.; Yang, S.; Song, L.; Dong, J.; Pan, X. Insight into the Promoting Role of Er Modification on SO2 Resistance for NH3-SCR at Low Temperature over FeMn/TiO2 Catalysts. Catalysts 2021, 11, 618. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050618
Du H, Han Z, Wu X, Li C, Gao Y, Yang S, Song L, Dong J, Pan X. Insight into the Promoting Role of Er Modification on SO2 Resistance for NH3-SCR at Low Temperature over FeMn/TiO2 Catalysts. Catalysts. 2021; 11(5):618. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050618
Chicago/Turabian StyleDu, Huan, Zhitao Han, Xitian Wu, Chenglong Li, Yu Gao, Shaolong Yang, Liguo Song, Jingming Dong, and Xinxiang Pan. 2021. "Insight into the Promoting Role of Er Modification on SO2 Resistance for NH3-SCR at Low Temperature over FeMn/TiO2 Catalysts" Catalysts 11, no. 5: 618. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050618