Bio-Catalytic Activity of Novel Mentha arvensis Intervened Biocompatible Magnesium Oxide Nanomaterials
Abstract
:1. Introduction
2. Results
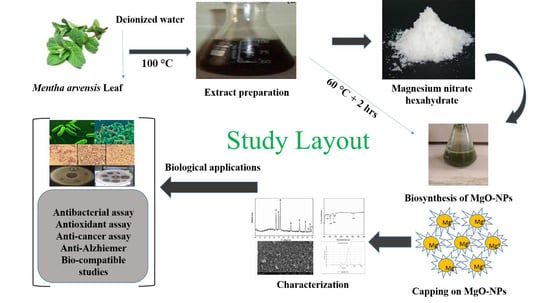
2.1. Biosynthesis of MgO-NPs
2.2. Optical Band Gap
2.3. Powder X-Ray Diffraction
2.4. Fourier Transformed Infrared Spectroscopy
2.5. Thermo Galvanometric Analysis
2.6. SEM, TEM and EDX Analysis of MgO-NPs
2.7. Zeta Size and Zeta Potential of MgO-NPs
2.8. Antibacterial Assay against H. pylori Bacterial Isolates
2.9. Protein Kinase Inhibition Assay
2.10. In Vitro AChE and BChE Inhibition Assays
2.11. In Vitro Cytotoxic Potential Against Hela Cell Lines
2.12. In Vitro Antioxidant Potential of MgO-NPs
2.13. Bio-Compatible Nature of MgO-NPs against Human (RBCs)
3. Discussion
4. Materials and Methods
4.1. Collection and Processing of the Plant Material
4.2. Biosynthesis of MgO Nanoparticles
4.3. Characterizations of Biosynthesized MgO-NPs
4.4. Antibacterial Assay of MgO-NPs against H. pylori Bacterial Strains
4.5. Protein Kinase Inhibition Assay
4.6. Anti-Alzheimer’s Activity
4.7. Anti-Cancer Potential of MgO-NPs against Hela Cell Lines
4.8. Estimation of Antioxidant Activity
4.8.1. DPPH Antioxidant Assay
4.8.2. Total Antioxidant Capacity Determination (TAC)
4.8.3. Total Reducing Power Determination (TRP)
4.8.4. Antioxidant ABTS Assay
4.9. Biocompatibility Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsden, J. Nanotechnology: An Introduction; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]
- Albrecht, M.A.; Evans, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-mediated green synthesis of iron nanoparticles. J. Nanoparticles 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Simonis, F.; Schilthuizen, S. Nanotechnology; Innovation Opportunities for Tomorrow’s Defence, Report; TNO Science & Industry Future Technology Center: Delft, The Netherlands, 2006. [Google Scholar]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Hasan, S. A review on nanoparticles: Their synthesis and types. Res. J. Recent Sci. 2015, 2277, 2502. [Google Scholar]
- Barzinjy, A.A.; Hamad, S.M.; Aydın, S.; Ahmed, M.H.; Hussain, F.H. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 11303–11316. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Fernández-García, M.; Rodriguez, J.A. Metal oxide nanoparticles. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Vergheese, M.; Vishal, S.K. Green synthesis of magnesium oxide nanoparticles using Trigonella foenum-graecum leaf extract and its antibacterial activity. J. Pharm. Phytochem. 2018, 7, 1193–1200. [Google Scholar]
- Bindhu, M.; Umadevi, M.; Micheal, M.K.; Arasu, M.V.; Al-Dhabi, N.A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- El-Moslamy, S.H. Bioprocessing strategies for cost-effective large-scale biogenic synthesis of nano-MgO from endophytic Streptomyces coelicolor strain E72 as an anti-multidrug-resistant pathogens agent. Sci. Rep. 2018, 8, 1–22. [Google Scholar] [CrossRef]
- Jeevanandam, J.; San Chan, Y.; Danquah, M.K. Biosynthesis and characterization of MgO nanoparticles from plant extracts via induced molecular nucleation. New J. Chem. 2017, 41, 2800–2814. [Google Scholar] [CrossRef]
- Thawkar, B.S. Phytochemical and pharmacological review of Mentha arvensis. Int. J. Green Pharm. (IJGP) 2016, 10, 2. [Google Scholar]
- do Nascimento, E.M.M.; Rodrigues, F.F.G.; Campos, A.R.; da Costa, J.G.M. Phytochemical prospection, toxicity and antimicrobial activity of Mentha arvensis (Labiatae) from northeast of Brazil. J. Young Pharm. 2009, 1, 210. [Google Scholar]
- Chan, K. Mentha Spicata-A Potential Cover Crop for Tropical Conservation Agriculture; University of Hawaii at Manoa: Honolulu, HI, USA, 2016. [Google Scholar]
- Zhao, B.T.; Kim, T.I.; Kim, Y.H.; Kang, J.S.; Min, B.S.; Son, J.K.; Woo, M.H. A comparative study of Mentha arvensis L. and Mentha haplocalyx Briq. by HPLC. Nat. Prod. Res. 2018, 32, 239–242. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Bhargava, R.; Khan, S. Superior dielectric properties and bandgap modulation in hydrothermally grown Gr/MgO nanocomposite. Phys. Lett. A 2019, 383, 1671–1676. [Google Scholar] [CrossRef]
- Wang, F.-H.; Chang, C.-L. Effect of substrate temperature on transparent conducting Al and F co-doped ZnO thin films prepared by rf magnetron sputtering. Appl. Surf. Sci. 2016, 370, 83–91. [Google Scholar] [CrossRef]
- Sushma, N.J.; Prathyusha, D.; Swathi, G.; Madhavi, T.; Raju, B.D.P.; Mallikarjuna, K.; Kim, H.-S. Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea—Characterization and in vitro antioxidant studies. Appl. Nanosci. 2016, 6, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Jayaseelan, C.; Ramkumar, R.; Rahuman, A.A.; Perumal, P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod. 2013, 45, 423–429. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Dang, H.H.; Vo, D.-V.N.; Bach, L.G.; Nguyen, T.D.; Van Tran, T. Biogenic synthesis of MgO nanoparticles from different extracts (flower, bark, leaf) of Tecoma stans (L.) and their utilization in selected organic dyes treatment. J. Hazard. Mater. 2021, 404, 124146. [Google Scholar] [CrossRef]
- Nandgaonkar, A.G. Bacterial Cellulose (BC) as a Functional Nanocomposite Biomaterial. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2014. [Google Scholar]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar—Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green synthesis and characterization of hexagonal shaped MgO nanoparticles using insulin plant (Costus pictus D. Don) leave extract and its antimicrobial as well as anticancer activity. Adv. Powder Technol. 2018, 29, 1685–1694. [Google Scholar] [CrossRef]
- Rahmani-Nezhad, S.; Dianat, S.; Saeedi, M.; Hadjiakhoondi, A. Synthesis, characterization and catalytic activity of plant-mediated MgO nanoparticles using Mucuna pruriens L. seed extract and their biological evaluation. J. Nanoanal. 2017, 4, 290–298. [Google Scholar]
- Wong, C.W.; San Chan, Y.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Abd Elkodous, M.; El-Sayyad, G.S. Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. J. Clust. Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Romero, C.D.; Chopin, S.F.; Buck, G.; Martinez, E.; Garcia, M.; Bixby, L. Antibacterial properties of common herbal remedies of the southwest. J. Ethnopharmacol. 2005, 99, 253–257. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, G.H.; Bradley, J.; Edwards, J.E., Jr.; Gilbert, D.; Scheld, M.; Bartlett, J.G. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 42, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B Biol. 2019, 197, 111531. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarov, T.; Kiran, B.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S. An overview of nanotechnology-based treatment approaches against Helicobacter Pylori. Expert Rev. Anti Infect. Ther. 2019, 17, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Shah, M.; Usman, H.; Khan, A.; Muhammad, Z.; Hano, C.; Abbasi, B.H. Biogenic Synthesis and Characterization of Antimicrobial and Anti-parasitic Zinc Oxide (ZnO) Nanoparticles using Aqueous Extracts of the Himalayan Columbine (Aquilegia pubiflora). Front. Mater. 2020, 7, 249. [Google Scholar] [CrossRef]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, H.; Khan, M.A.; Usman, H.; Shah, M.; Ansir, R.; Faisal, S.; Ullah, N.; Rahman, L. The Aquilegia pubiflora (Himalayan columbine) mediated synthesis of nanoceria for diverse biomedical applications. RSC Adv. 2020, 10, 19219–19231. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.T.; Ayaz, M.; Ovais, M.; Wadood, A.; Ali, M.; Shinwari, Z.K.; Maaza, M. In vitro cholinesterase enzymes inhibitory potential and in silico molecular docking studies of biogenic metal oxides nanoparticles. Inorg. Nano Met. Chem. 2018, 48, 441–448. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Supp. 1), 693–707. [Google Scholar] [CrossRef] [Green Version]
- Šinko, G.; Vrček, I.V.; Goessler, W.; Leitinger, G.; Dijanošić, A.; Miljanić, S. Alteration of cholinesterase activity as possible mechanism of silver nanoparticle toxicity. Environ. Sci. Pollut. Res. 2014, 21, 1391–1400. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753. [Google Scholar]
- Safaepour, M.; Shahverdi, A.R.; Shahverdi, H.R.; Khorramizadeh, M.R.; Gohari, A.R. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J. Med Biotechnol. 2009, 1, 111. [Google Scholar] [PubMed]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef]
- Sergiev, I.; Todorova, D.; Shopova, E.; Jankauskiene, J.; Jankovska-Bortkevič, E.; Jurkonienė, S. Exogenous auxin type compounds amend PEG-induced physiological responses of pea plants. Sci. Hortic. 2019, 248, 200–205. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A. Changes in antioxidants potential, secondary metabolites and plant hormones induced by different fungicides treatment in cotton plants. Pestic. Biochem. Physiol. 2017, 142, 117–122. [Google Scholar] [CrossRef]
- Rehman, M.; Ullah, S.; Bao, Y.; Wang, B.; Peng, D.; Liu, L. Light-emitting diodes: Whether an efficient source of light for indoor plants? Environ. Sci. Pollut. Res. 2017, 24, 24743–24752. [Google Scholar] [CrossRef]
- Prieto, M.; Curran, T.P.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef] [Green Version]
- Skonieczna, M.; Hudy, D. Biological activity of Silver Nanoparticles and their applications in Anticancer Therapy. In Biological Activity of Silver Nanoparticles and Their Applications in Anticancer Therapy; IntechOpen: London, UK, 2018; p. 131. [Google Scholar]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Ahmad, M.S.; Mirza, B. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. IJPR 2012, 11, 241. [Google Scholar] [PubMed]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasar, M.Q.; Khalil, A.T.; Ali, M.; Shah, M.; Ayaz, M.; Shinwari, Z.K. Phytochemical analysis, Ephedra Procera CA Mey. Mediated green synthesis of silver nanoparticles, their cytotoxic and antimicrobial potentials. Medicina 2019, 55, 369. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar] [PubMed]
- Vijayan, S.R.; Santhiyagu, P.; Ramasamy, R.; Arivalagan, P.; Kumar, G.; Ethiraj, K.; Ramaswamy, B.R. Seaweeds: A resource for marine bionanotechnology. Enzym. Microb. Technol. 2016, 95, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Daima, H.K.; Kachhwaha, S.; Kothari, S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig. J. Nanomater. Biostruct. 2009, 4, 557–563. [Google Scholar]
- Zhang, T.; Ye, J.; Xue, C.; Wang, Y.; Liao, W.; Mao, L.; Yuan, M.; Lian, S. Structural characteristics and bioactive properties of a novel polysaccharide from Flammulina velutipes. Carbohydr. Polym. 2018, 197, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Shah, S.; Muhammad, Z.; Shah, S.A.; Faisal, S.; Khattak, U.; ul Haq, T.; Akbar, M.T. In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres. Green Process. Synth. 2021, 10, 101–111. [Google Scholar] [CrossRef]
- Faisal, S.; Khan, M.A.; Jan, H.; Shah, S.A.; Shah, S.; Rizwan, M.; Ullah, W.; Akbar, M.T. Edible mushroom (Flammulina velutipes) as biosource for silver nanoparticles: From synthesis to diverse biomedical and environmental applications. Nanotechnology 2020, 32, 065101. [Google Scholar] [CrossRef] [PubMed]
- Nasr, H.; Nassar, O.; El-Sayed, M.; Kobisi, A. Characterization and antimicrobial activity of lemon peel mediated green synthesis of silver nanoparticles. Int. J. Biol. Chem. 2020, 12, 56–63. [Google Scholar] [CrossRef]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle characterization: What to measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef]
- Jebali, A.; Kazemi, B. Nano-based antileishmanial agents: A toxicological study on nanoparticles for future treatment of cutaneous leishmaniasis. Toxicol. In Vitro 2013, 27, 1896–1904. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.; Shim, Y.Y.; Reaney, M.J.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Shah, S.A.; Faisal, S.; Khan, A.; Ullah, R.; Ali, N.; Bilal, M. Engineering novel gold nanoparticles using Sageretia thea leaf extract and evaluation of their biological activities. J. Nanostruct. Chem. 2021, 1–12. [Google Scholar] [CrossRef]
- Suresh, J.; Yuvakkumar, R.; Sundrarajan, M.; Hong, S.I. Green Synthesis of Magnesium Oxide Nanoparticles. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2014; pp. 141–144. [Google Scholar]
- Shah, R.; Shah, S.A.; Shah, S.; Faisal, S.; Ullah, F. Green Synthesis and Antibacterial Activity of Gold Nanoparticles of Digera muricata. Indian J. Pharm. Sci. 2020, 82, 374–378. [Google Scholar] [CrossRef]
- Barzinjy, A.; Mustafa, S.; Ismael, H. Characterization of ZnO NPs prepared from green synthesis using Euphorbia Petiolata leaves. EAJSE 2019, 4, 74–83. [Google Scholar]
- Morais, M.; Namouni, F. Asteroids in retrograde resonance with Jupiter and Saturn. Mon. Not. R. Astron. Soc. Lett. 2013, 436, L30–L34. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Jan, H.; Faisal, S.; Shah, S.A.; Shah, S.; Khan, M.N.; Akbar, M.T.; Rizwan, M.; Jan, F.; Syed, S. In vitro Examination of Anti-parasitic, Anti-Alzheimer, Insecticidal and Cytotoxic Potential of Ajuga Bracteosa Wallich Leaves Extracts. Saudi J. Biol. Sci. 2021, 28, 3031–3036. [Google Scholar] [CrossRef]
- Faisal, S.; Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Akhtar, N.; et al. (2021 Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous2 Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 14, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Kainat; Khan, M.A.; Ali, F.; Faisal, S.; Rizwan, M.; Hussain, Z.; Zaman, N.; Afsheen, Z.; Uddin, M.N.; Bibi, N. Exploring the therapeutic potential of Hibiscus rosa sinensis synthesized cobalt oxide (Co3O4-NPs) and magnesium oxide nanoparticles (MgO-NPs). Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Faisal, S.; Shah, S.A.; Shah, S.; Akbar, M.T.; Jan, F.; Haq, I.; Baber, M.E.; Aman, K.; Zahir, F.; Bibi, F.; et al. In Vitro Biomedical and Photo-Catalytic Application of Bio-Inspired Zingiber officinale Mediated Silver Nanoparticles. J. Biomed. Nanotechnol. 2020, 16, 492–504. [Google Scholar] [CrossRef]







| H. pylori Strains | MgO-NPs | |||
|---|---|---|---|---|
| 5 mg/mL | 4 mg/mL | 2 mg/mL | 1 mg/mL | |
| Helicobacter felis | 17.19 ± 0.83 * | 13.32 ± 0.53 * | 9.55 ± 0.56 * | 6.23 ± 0.31 * |
| Helicobacter suis | 16.49 ± 0.64 ** | 13.62 ± 0.51 * | 9.29 ± 0.53 * | 5.48 ± 0.37 ** |
| Helicobacter salomonis | 16.09 ± 0.66 ** | 9.92 ± 0.42 *** | 7.70 ± 0.49 *** | 5.71 ± 0.23 ** |
| Helicobacter bizzozeronii | 14.19 ± 0.51 ** | 10.23 ± 0.59 ** | 8.43 ± 0.41 ** | 4.79 ± 0.26 *** |
| Positive control (Kanamycin) | 21.82 ± 0.74 | 16.71 ± 0.82 | 12.67 ± 0.58 | 12.14 ± 0.44 |
| Conc. (µg/mL) | TAC (µg AAE/mg) | TRP (µg AAE/mg) | ABTS (TEAC) | DPPH (%FRSA) |
|---|---|---|---|---|
| 400 | 61.1 ± 0.73 | 43.41 ± 0.23 | 77.12 ± 0.18 | 56.3 ± 0.38 |
| 200 | 55.37 ± 0.17 | 39.51 ± 0.47 | 63.63 ± 0.29 | 41.1 ± 0.61 |
| 100 | 33.86 ± 0.62 | 22.23 ± 0.16 | 44.64 ± 0.46 | 27.69 ± 0.42 |
| 50 | 25.29 ± 0.56 | 16.76 ± 0.28 | 25.47 ± 0.16 | 18.45 ± 0.88 |
| 25 | 19.16 ± 0.15 | 10.41 ± 0.86 | 16.39 ± 0.25 | 10.19 ± 0.38 |
| S. No | Conc. μg/mL | % Hemolysis |
|---|---|---|
| 1 | 400 | 2.11 ± 0.13 |
| 2 | 200 | 2.06 ± 0.11 |
| 3 | 100 | 1.19 ± 0.09 |
| 4 | 50 | 0.83 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, S.; Abdullah; Jan, H.; Shah, S.A.; Shah, S.; Rizwan, M.; Zaman, N.; Hussain, Z.; Uddin, M.N.; Bibi, N.; et al. Bio-Catalytic Activity of Novel Mentha arvensis Intervened Biocompatible Magnesium Oxide Nanomaterials. Catalysts 2021, 11, 780. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070780
Faisal S, Abdullah, Jan H, Shah SA, Shah S, Rizwan M, Zaman N, Hussain Z, Uddin MN, Bibi N, et al. Bio-Catalytic Activity of Novel Mentha arvensis Intervened Biocompatible Magnesium Oxide Nanomaterials. Catalysts. 2021; 11(7):780. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070780
Chicago/Turabian StyleFaisal, Shah, Abdullah, Hasnain Jan, Sajjad Ali Shah, Sumaira Shah, Muhammad Rizwan, Nasib Zaman, Zahid Hussain, Muhammad Nazir Uddin, Nadia Bibi, and et al. 2021. "Bio-Catalytic Activity of Novel Mentha arvensis Intervened Biocompatible Magnesium Oxide Nanomaterials" Catalysts 11, no. 7: 780. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11070780