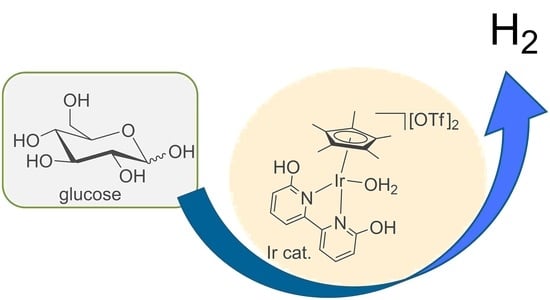
Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedures for the Hydrogen Production from D-Glucose Catalyzed by Various Iridium Complexes
3.3. Procedure for the Simultaneous Parallel Experiment (Hydrogenation of 1-Decene with Hydrogen Produced by the Dehydrogenation of Glucose)
3.4. General Procedures for the Hydrogen Production from Various Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martín, A.J.; Shinagawa, T.; Pérez-Ramírez, J. Electrocatalytic Reduction of Nitrogen: From Haber-Bosch to Ammonia Artificial Leaf. Chem 2019, 5, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber-Bosch Ammonia in a Carbon-free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A Review on the Catalytic Conversion of CO2 Using H2 for Synthesis of CO, Methanol, and Hydrocarbons. J. CO2 Util. 2021, 44, 10413. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic Reduction of CO2 by H2 for Synthesis of CO, Methanol and Hydrocarbons: Challenges and Opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef]
- Alves, J.J.; Towler, G.P. Analysis of Refinery Hydrogen Distribution Systems. Ind. Eng. Chem. Res. 2002, 41, 5759–5769. [Google Scholar] [CrossRef]
- Song, C. An Overview of New Approaches to Deep Desulfurization for Ultra-clean Gasoline, Diesel Fuel and Jet Fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and Technology of Novel Processes for Deep Desulfurization of Oil Refinery Streams: A Review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Hydrogen Industry Applications: Past, Present, and Future. Available online: https://wha-international.com/hydrogen-in-industry/ (accessed on 25 June 2021).
- Dunn, S. Hydrogen Futures: Toward a Sustainable Energy System. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen Nexus in a Sustainable Energy Future. Energy Environ. Sci. 2008, 1, 79–85. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.-J.; Chen, J.; Zhao, Y.-R.; Chang, J.; Junge, H.; Beller, M.; Li, Y. Streamlined Hydrogen Production from Biomass. Nat. Catal. 2018, 1, 332–338. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.J.; Pant, K.K.; Gupta, R.B. Hydrogen Production from Glucose Using Ru/Al2O3 Catalyst in Supercritical Water. Ind. Eng. Chem. Res. 2007, 46, 3574–3579. [Google Scholar] [CrossRef]
- Behnia, I.; Yuan, Z.; Charpentier, P.; Xu, C. Production of Methane and Hydrogen via Supercritical Water Gasification of Renewable Glucose at a Relatively Low Temperature: Effects of Metal Catalysts and Supports. Fuel Process. Technol. 2016, 143, 27–34. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Clowdhury, M.B.I.; Jhawar, A.K.; Charpentier, P.A. Supercritical Water Gasification of Glucose Using Bimetallic Aerogel Ru-Ni-Al2O3 Catalyst for H2 Production. Biomass Bioenergy 2017, 107, 39–51. [Google Scholar] [CrossRef]
- Sharma, K. Carbohydrate-to-Hydrogen Production Technologies: A Mini-Review. Renew. Sustain. Energy Reviews. 2019, 105, 138–143. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Hopkins, R.C.; Adams, M.W.W.; Evans, B.R.; Mielenz, J.R.; Zhang, Y.-H.P. Spontaneous High-Yield Production of Hydrogen from Cellulosic Materials and Water Catalyzed by Enzyme Cocktails. ChemSusChem 2009, 2, 149–152. [Google Scholar] [CrossRef]
- Borja, P.; Vicent, C.; Baya, M.; Garcia, H.; Mata, J.A. Iridium Complexes Catalysed the Selective Dehydrogenation of Glucose to Gluconic Acid in Water. Green Chem. 2018, 20, 4094–4101. [Google Scholar] [CrossRef] [Green Version]
- Taccardi, N.; Assenbaum, D.; Berger, M.E.M.; Bösmann, A.; Enzenberger, F.; Wölfel, R.; Neuendorf, S.; Goeke, V.; Schödel, N.; Maass, H.-J.; et al. Catalytic Production of Hydrogen from Glucose and Other Carbohydrates under Exceptionally Mild Reaction Conditions. Green Chem. 2010, 12, 1150–1156, Hydrogen production from glucose in ionic liquid has been also reported. [Google Scholar]
- Zhan, Y.; Shen, Y.; Li, S.; Yue, B.; Zhou, X. Hydrogen Generation from Glucose Catalyzed by Organoruthenium Catalysts under Mild Conditions. Chem. Commun. 2017, 53, 4230–4233, Hydrogen production under acidic conditions has been also reported. [Google Scholar]
- Fujita, K. Development and Application of New Iridium Catalysts for Efficient Dehydrogenative Reactions of Organic Molecules. Bull. Chem. Soc. Jpn. 2019, 92, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2012, 134, 3643–3646. [Google Scholar] [CrossRef]
- Fujita, K.; Ito, W.; Yamaguchi, R. Dehydrogenative Lactonization of Diols in Aqueous Media Catalyzed by a Water-Soluble Iridium Complex Bearing a Functional Bipyridine Ligand. ChemCatChem 2014, 6, 109–112. [Google Scholar] [CrossRef]
- The yield of hydrogen in this paper is expressed as 100% when 1 equiv of hydrogen molecules are generated from 1 equiv of glucose.
- Ball, R.G.; Graham, W.A.G.; Heinekey, D.M.; Hoyano, J.K.; McMaster, A.D.; Mattson, B.M.; Michel, S.T. Synthesis and Structure of [η5-C5Me5)Ir(CO)]2. Inorg. Chem. 1990, 29, 2023–2025. [Google Scholar] [CrossRef]
- Ogo, S.; Makihara, N.; Watanabe, Y. pH-Dependent Transfer Hydrogenation of Water-Soluble Carbonyl Compounds with [Cp*IrIII(H2O)3]2+ (Cp* = η5-C5Me5) as a Catalyst Precursor and HCOONa as a Hydrogen Donor in Water. Organometallics 1999, 18, 5470–5474. [Google Scholar] [CrossRef]
- Xiao, X.-Q.; Jin, G.-X. Functionalized N-Heterocyclic Carbene Iridium Complexes: Synthesis, Structure and Addition Polymerization of Norbornene. J. Organomet. Chem. 2008, 693, 3363–3368. [Google Scholar] [CrossRef]
- Ogo, S.; Makihara, N.; Kaneko, Y.; Watanabe, Y. pH-Dependent Transfer Hydrogenation, Reductive Amination, and Dehalogenation of Water-Soluble Carbonyl Compounds and Alkyl Halides Promoted by Cp*Ir Complexes. Organometallics 2001, 20, 4903–4910. [Google Scholar] [CrossRef]
- Himeda, Y.; Onozawa-Komatsuzaki, N.; Miyazawa, S.; Sugihara, H.; Hirose, T.; Kasuga, K. pH-Dependent Catalytic Activity and Chemoselectivity in Transfer Hydrogenation Catalyzed by Iridium Complex with 4,4′-Dihydroxy-2,2′-bipyridine. Chem. Eur. J. 2008, 14, 11076–11081. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Glazebnik, S.; Zhao, K. Peralkylation of Saccharides under Aqueous Conditions. Tetrahedron Lett. 1995, 36, 2953–2956. [Google Scholar] [CrossRef]
- Xu, G.; Moeller, K.D. Anodic Coupling Reactions and the Synthesis of C-Glycosides. Org. Lett. 2010, 12, 2590–2593. [Google Scholar] [CrossRef]
- Collins, D.J.; Hibberd, A.I.; Skelton, B.W.; White, A.H. Enolic Ortho Esters VII: Involvement of Magnesium Halides as Lewis Acids in the Reaction of Grignard Reagents with 1,6-Dideoxy-1,1,-ethylenedioxy-2,3,4-tri-O-methyl-D-xylo-hex-5-enopyranose and its 6-Phenyl Derivative: A Correction. Aust. J. Chem. 1998, 51, 681–694. [Google Scholar] [CrossRef]
- Bihovsky, R.; Selick, C.; Giusti, I. Synthesis of C-Glucosides by Reaction of Glucosyl Halides with Organocuprates. J. Org. Chem. 1988, 53, 4026–4031. [Google Scholar] [CrossRef]






| Entry | Catalyst (mol %) | Yield of Hydrogen (%) a |
|---|---|---|
| 1 | 1 (0.20 mol %) | 7 |
| 2 | 2 (0.20 mol %) | 76 |
| 3 | [Cp*IrCl2]2 (0.20 mol % Ir) | 0 |
| 4 | [Cp*Ir(H2O)3][OTf]2 (0.20 mol %) | trace |
| 5 | 3 (0.20 mol %) | trace |
| 6 | 4 (0.20 mol %) | trace |
| 7 | 5 (0.20 mol %) | 71 |
| 8 | 2 (1.0 mol %) | 95 |

| Entry | Substrate | Yield of Hydrogen (%) a |
|---|---|---|
| 1 |  | 95 |
| 2 |  | 8 |
| 3 |  | 14 |
| 4 |  | 0 |
| 5 |  | 92 |

| Entry | Substrate | Yield of Hydrogen (%)a |
|---|---|---|
| 1 |  | 95 |
| 2 |  | 87 |
| 3 |  | 83 |
| 4 |  | 83 |
| 5 |  | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, K.-i.; Inoue, T.; Tanaka, T.; Jeong, J.; Furukawa, S.; Yamaguchi, R. Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides. Catalysts 2021, 11, 891. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080891
Fujita K-i, Inoue T, Tanaka T, Jeong J, Furukawa S, Yamaguchi R. Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides. Catalysts. 2021; 11(8):891. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080891
Chicago/Turabian StyleFujita, Ken-ichi, Takayoshi Inoue, Toshiki Tanaka, Jaeyoung Jeong, Shohichi Furukawa, and Ryohei Yamaguchi. 2021. "Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides" Catalysts 11, no. 8: 891. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080891