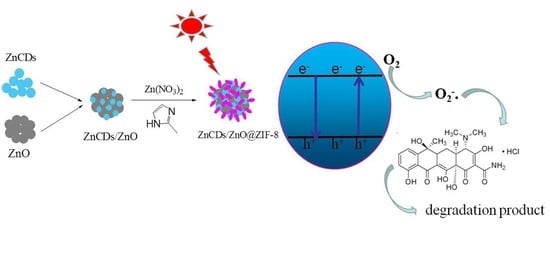
ZnCDs/ZnO@ZIF-8 Zeolite Composites for the Photocatalytic Degradation of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of ZnCDs/ZnO@ZIF-8
2.2. Characterization of ZnCDs/ZnO@ZIF-8
2.3. Catalytic Performance of ZnCDs/ZnO@ZIF-8
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of ZnCDs
3.4. Synthesis of ZnO
3.5. Synthesis of ZnCDs/ZnO
3.6. Synthesis of ZnCDs/ZnO@ZIF-8
3.7. Photocatalytic Degradation of Tetracycline
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, X.; Liu, X. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, S.Z.; Hu, Y.M.; Wang, J.L. Biodegradation of typical pharmaceutical compounds by a novel strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, J.; Fan, Y.; Zhang, S.; Dai, W. A novel multifunctional p-type semiconductor@MOFs nanoporous platform for simultaneous sensing and photodegradation of tetracycline. ACS Appl. Mater. Interfaces 2020, 12, 11036–11044. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Lai, J.; Li, Y.; Yang, J.; Cui, S.; Li, Y. The degradation of tetracycline by modified BiOCl nanosheets with carbon dots from the chlorella. J. Alloys Compd. 2021, 855, 157454. [Google Scholar] [CrossRef]
- Ponraj, Y.K.; Borah, B. Separation of methane from ethane and propane by selective adsorption and diffusion in MOF Cu-BTC: A molecular simulation study. J. Mol. Graph. Modell. 2020, 97, 107574. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, X.J.; Liu, Y.; Cheng, M.; Liu, Z.F.; Zeng, G.M.; Shao, B.B.; Liang, Q.H.; Zhang, W.; He, Q.Y.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, Z.; Su, S.; Song, S.; Xu, T.; Li, Z.; Zhou, W. Recent advances in metal organic frame photocatalysts for environment and energy applications. Appl. Mater. Today 2020, 21, 100821. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.-Y.; Wang, Y.-H.; Wang, D.-D.; Song, Z.; Zhang, C.-D.; Wang, H.-S. Colorable zeolitic imidazolate frameworks for colorimetric detection of biomolecules. Anal. Chem. 2020, 92, 12670–12677. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Weber, M.D.R.; Baker, T.L.; Dao, B.; Kwon, C.; Tian, F.Y. Exploring the aggregative growth of nanoporous zeolitic imidazolate framework ZIF-8. Cryst. Growth Des. 2020, 20, 2305–2312. [Google Scholar] [CrossRef]
- Wolf, A.; Diestel, L.; Lubkemann, F.; Kodanek, T.; Mohamed, T.; Caro, J.; Dorfs, D. Plasmonic semiconductor nanoparticles in a metal−organic framework structure and their in situ cation exchange. Chem. Mater. 2016, 28, 7511–7518. [Google Scholar] [CrossRef]
- Jian, M.P.; Wang, H.; Liu, R.P.; Qu, J.H.; Wang, H.T.; Zhang, X.W. Self-assembled one-dimensional MnO2@zeolitic imidazolate framework-8 nanostructures for highly efficient arsenite removal. Environ. Sci. Nano 2016, 3, 1186–1194. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) reduction and immobilization by core-double-shell structured magnetic polydopamine@zeolitic idazolate frameworks-8 microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Esken, D.; Turner, S.; Wiktor, C.; Kalidindi, S.B.; Van Tendeloo, G.; Fischer, R.A. GaN@ZIF-8: Selective formation of gallium nitride quantum dots. inside a zinc methylimidazolate framework. J. Am. Chem. Soc. 2011, 133, 16370–16373. [Google Scholar] [CrossRef]
- Wang, X.B.; Liu, J.; Leong, S.; Lin, X.C.; Wei, J.; Kong, B.; Xu, Y.F.; Low, Z.-X.; Yao, J.F.; Wang, H.T. Rapid construction of ZnO@ZIF-8 heterostructures with size-selective photocatalysis properties. ACS Appl. Mater. Interface 2016, 8, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, F.; Dong, W.; Hou, J.; Lu, P.; Gong, J. Self-template synthesis of core–shell ZnO@ZIF-8 nanospheres and the photocatalysis under UV irradiation. Mater. Lett. 2015, 156, 50–53. [Google Scholar] [CrossRef]
- Zhan, W.-W.; Kuang, Q.; Zhou, J.-Z.; Kong, X.-J.; Xie, Z.-X.; Zheng, L.-S. Semiconductor@metal-organic framework core-shell heterostructures: A case of ZnO@ZIF-8 nanorods with selective photoelectrochemical response. J. Am. Chem. Soc. 2013, 135, 1926–1933. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.J.; Lu, S.Y.; Song, Y.B.; Feng, T.L.; Tao, S.Y.; Liu, J.J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L.Y. Recent progress of carbon dot precursors and photocatalysis applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [Green Version]
- Phang, S.J.; Tan, L.-L. Recent advances in carbon quantum dot (CQD)-based two dimensional materials for photocatalytic applications. Catal. Sci. Technol. 2019, 9, 5882–5905. [Google Scholar] [CrossRef]
- Hazarika, D.; Karak, N. Photocatalytic degradation of organic contaminants under solar light using carbon dot/titanium dioxide nanohybrid, obtained through a facile approach. Appl. Surf. Sci. 2016, 376, 276–285. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, L.; Gu, H.; Li, Z.; Wang, Y.; Luo, Q.; Yang, Y.; Liu, X.; Wang, H.; Ma, C.-Q. Zinc oxide coated carbon dot nanoparticles as electron transport layer for inverted polymer solar cells. ACS Appl. Energy Mater. 2020, 3, 11388–11397. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, J.-K.; Wang, J.-D.; Yang, X.-H. Mass production, enhanced visible light photocatalytic efficiency, and application of modified ZnO nanocrystals by carbon dots. Ind. Eng. Chem. Res. 2015, 54, 1766–1772. [Google Scholar] [CrossRef]
- Chin, M.; Cisneros, C.; Araiza, S.M.; Vargas, K.M.; Ishihara, K.M.; Tian, F. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8). RSC Adv. 2018, 8, 26987–26997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ge, S.; Cheng, W.; Hu, Z.; Shao, Q.; Wang, X.; Lin, J.; Dong, M.; Wang, J.; Guo, Z. Microwave hydrothermally synthesized metal−organic framework-5 derived C-doped ZnO with enhanced photocatalytic degradation of Rhodamine B. Langmuir 2020, 36, 9658–9667. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, T.; Liu, H.; Qiu, J.; Zhang, X. In situ fabrication of a perfect Pd/ZnO@ZIF-8 core-shell microsphere as an efficient catalyst by a ZnO support-induced ZIF-8 growth strategy. Nanoscale 2015, 7, 7615–7623. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.; Meng, T.; Lee, C.; Wang, P.; Zhang, W. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Li, Y. A Ternary g-C3N4/Pt/ZnO photoanode for efficient photoelectrochemical water splitting. Int. J. Hydrogen Energy 2015, 40, 9080–9087. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. Type-II heterostructure of ZnO and carbon dots demonstrates enhanced photoanodic performance in photoelectrochemical water splitting. Inorg. Chem. 2020, 59, 6988–6999. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. Near-field and far-field plasmonic effects of gold nanoparticles decorated on ZnO nanosheets for enhanced solar water splitting. ACS Appl. Nano Mater. 2020, 3, 1153–1165. [Google Scholar] [CrossRef]
- Biesingera, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Gelb, L.D.; Gubbins, K.E. Pore size distributions in porous glasses: A computer simulation study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Cheng, Y.; Mei, Y.; Deng, S.Y.; Li, J. In situ synthesis and photocatalytic performance of three dimensional composites CdS@DMSA-GO. Chin. J. Inorg. Chem. 2020, 36, 714–729. [Google Scholar]
- Li, Y.; Jiang, Y.; Ruan, Z.; Lin, K.; Yu, Z.; Zheng, Z.; Xu, X.; Yuan, Y. Simulation-guided synthesis of graphitic carbon nitride beads with 3D interconnected and continuous meso/macropore channels for enhanced light absorption and photocatalytic performance. J. Mater. Chem. A 2017, 5, 21300–21312. [Google Scholar] [CrossRef]
- Bian, S.; Zhou, C.; Li, P.; Liu, J.; Dong, X.; Xi, F. Graphene quantum dots decorated titania nanosheets heterojunction efficient charge separation and enhanced visible-light photocatalytic performance. ChemCatChem 2017, 9, 3349–3357. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.B.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type ⅡAgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liu, N.; Zhang, X.D.; Wu, M.H.; Tang, L. Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Yuan, D.; Ding, J.; Zhou, J.; Wang, L.; Wan, H.; Dai, W.-L.; Guan, G. Graphite carbon nitride nanosheets decorated with ZIF-8 nanoparticles: Effects of the preparation method and their special hybrid structures on the photocatalytic performance. J. Alloys Compd. 2018, 762, 98–108. [Google Scholar] [CrossRef]
- Liang, X.; Quan, B.; Ji, G.; Liu, W.; Zhao, H.; Dai, S.; Lv, J.; Du, Y. Tunable dielectric performance derived from the metal−organic framework/reduced graphene oxide hybrid with broadband absorption. ACS Sustain. Chem. Eng. 2017, 5, 10570–10579. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Hoper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai, China. J. Hazard. Mater. 2012, 235, 178–185. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Wang, X.; Mei, Y.; Wang, D.; Ji, C. ZnCDs/ZnO@ZIF-8 Zeolite Composites for the Photocatalytic Degradation of Tetracycline. Catalysts 2021, 11, 934. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080934
Cheng Y, Wang X, Mei Y, Wang D, Ji C. ZnCDs/ZnO@ZIF-8 Zeolite Composites for the Photocatalytic Degradation of Tetracycline. Catalysts. 2021; 11(8):934. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080934
Chicago/Turabian StyleCheng, Yong, Xiuxiu Wang, Yu Mei, Dan Wang, and Changchun Ji. 2021. "ZnCDs/ZnO@ZIF-8 Zeolite Composites for the Photocatalytic Degradation of Tetracycline" Catalysts 11, no. 8: 934. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080934