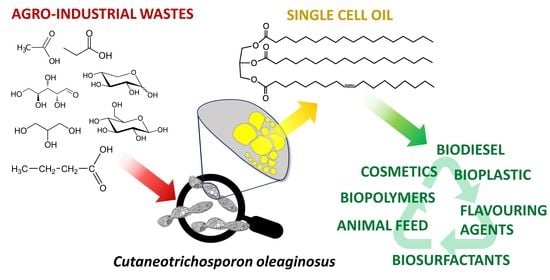
Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes
Abstract
:1. Introduction
2. Taxonomy of Biocatalyst
3. Applications of C. oleaginosus in the Bioconversion of Agro-Industrial Wastes to Single-Cell Oils
3.1. Acetic Acid and Volatile Organic Acids
3.2. Crude Glycerol
3.3. N-Acetylglucosamine
3.4. Lignocellulosic Biomasses
3.5. Wastepaper
4. Applications of Single-Cell Oils, Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Bari, I.; Cuna, D.; Di Fidio, N. Biofuels, Biochemicals, and Bioproducts. In Biofuels Production and Processing Technology, 1st ed.; Riazi, M.R., Chiaramonti, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 533–568. [Google Scholar]
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and biomass-based energy supply and demand. New Biotechnol. 2020, 60, 76–84. [Google Scholar] [CrossRef]
- Di Fidio, N.; Dragoni, F.; Antonetti, C.; De Bari, I.; Raspolli Galletti, A.M.; Ragaglini, G. From paper mill waste to single cell oil: Enzymatic hydrolysis to sugars and their fermentation into microbial oil by the yeast Lipomyces starkeyi. Bioresour. Technol. 2020, 315, 123790–123799. [Google Scholar] [CrossRef] [PubMed]
- Di Fidio, N.; Ragaglini, G.; Dragoni, F.; Antonetti, C.; Raspolli Galletti, A.M. Integrated cascade biorefinery processes for the production of single cell oil by Lipomyces starkeyi from Arundo donax L. hydrolysates. Bioresour. Technol. 2021, 325, 124635–124645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Das, P.K.; Das, B.P.; Dash, P. Role of energy crops to meet the rural energy needs: An overview. In Biomass Valorization to Bioenergy, 1st ed.; Kumar, R.P., Bharathiraja, B., Kataki, R., Moholkar, V.S., Eds.; Springer: Berlin, Germany, 2020; Volume 1, pp. 11–30. [Google Scholar]
- Caporusso, A.; De Bari, I.; Valerio, V.; Albergo, R.; Liuzzi, F. Conversion of cardoon crop residues into single cell oils by Lipomyces tetrasporus and Cutaneotrichosporon curvatus: Process optimizations to overcome the microbial inhibition of lignocellulosic hydrolysates. Ind. Crops Prod. 2021, 159, 113030–113039. [Google Scholar] [CrossRef]
- De Bari, I.; Liuzzi, F.; Ambrico, A.; Trupo, M. Arundo donax Refining to Second Generation Bioethanol and Furfural. Processes 2020, 8, 1591. [Google Scholar] [CrossRef]
- Antonetti, C.; Gori, S.; Licursi, D.; Pasini, G.; Frigo, S.; López, M.; Parajó, J.C.; Raspolli Galletti, A.M. One-pot alcoholysis of the lignocellulosic Eucalyptus nitens biomass to n-butyl levulinate, a valuable additive for diesel motor fuel. Catalysts 2020, 10, 509. [Google Scholar] [CrossRef]
- D’Espaux, L.; Mendez-Perez, D.; Li, R.; Keasling, J.D. Synthetic biology for microbial production of lipid-based biofuels. Curr. Opin. Chem. Biol. 2015, 29, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murguía-Ortiz, D.; Cordova, I.; Manriquez, M.; Ortiz-Islas, E.; Cabrera-Sierra, R.; Contreras, J.; Alcántar-Vázquez, B.; Trejo-Rubio, M.; Vázquez-Rodríguez, J.; Castro, L. Na-CaO/MgO dolomites used as heterogeneous catalysts in canola oil transesterification for biodiesel production. Mater. Lett. 2021, 291, 129587–129590. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Investigation of ultrasound-assisted KOH and CaO catalyzed transesterification for biodiesel production from waste cotton-seed cooking oil: Process optimization and conversion rate evaluation. J. Clean. Prod. 2020, 259, 120982–121001. [Google Scholar] [CrossRef]
- Khoobbakht, G.; Kheiralipour, K.; Yuan, W.; Seifi, M.R.; Karimi, M. Desirability function approach for optimization of enzymatic transesterification catalyzed by lipase immobilized on mesoporous magnetic nanoparticles. Renew. Energy 2020, 158, 253–262. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic biomass as a substrate for oleaginous microorganisms: A review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Xiong, X.; Xia, Y.; Qiao, J. Physiology, Application, and Bioengineering of Oleaginous Microorganisms. Front. Microbiol. 2021, 12, 1060–1062. [Google Scholar] [CrossRef]
- Di Fidio, N.; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; De Bari, I. Single cell oil production from undetoxified Arundo donax L. hydrolysate by Cutaneotrichosporon curvatus. J. Microbiol. Biotechnol. 2019, 29, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probst, K.V.; Schulte, L.R.; Durrett, T.P.; Rezac, M.E.; Vadlani, P.V. Oleaginous yeast: A value-added platform for renewable oils. Crit. Rev. Biotechnol. 2016, 36, 942–955. [Google Scholar] [CrossRef]
- Blomqvist, J.; Pickova, J.; Tilami, S.K.; Sampels, S.; Mikkelsen, N.; Brandenburg, J.; Sandgren, M.; Passoth, V. Oleaginous yeast as a component in fish feed. Sci. Rep. 2018, 8, 15945. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—from biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, A.; Maiti, M.K. Lipid production by oleaginous yeasts. In Advances in Applied Microbiology, 1st ed.; Elsevier: Cambridge, MA, USA, 2021; Volume 116, pp. 1–98. [Google Scholar]
- Crognale, S.; Liuzzi, F.; D’Annibale, A.; De Bari, I.; Petruccioli, M. Cynara cardunculus a novel substrate for solid-state production of Aspergillus tubingensis cellulases and sugar hydrolysates. Biomass Bioenergy 2019, 127, 105276–105284. [Google Scholar] [CrossRef]
- Hashem, A.H.; Hasanin, M.S.; Khalil, A.M.A.; Suleiman, W.B. Eco-green conversion of watermelon peels to single cell oils using a unique oleaginous fungus: Lichtheimia corymbifera AH13. Waste Biomass Valorization 2020, 11, 5721–5732. [Google Scholar] [CrossRef]
- Liuzzi, F.; Mastrolitti, S.; De Bari, I. Hydrolysis of corn stover by Talaromyces cellulolyticus enzymes: Evaluation of the residual enzymes activities through the process. Appl. Biochem. Biotechnol. 2019, 188, 690–705. [Google Scholar] [CrossRef]
- Juanssilfero, A.B.; Kahar, P.; Amza, R.L.; Miyamoto, N.; Otsuka, H.; Matsumoto, H.; Kihira, C.; Thontowi, A.; Ogino, C.; Prasetya, B. Selection of oleaginous yeasts capable of high lipid accumulation during challenges from inhibitory chemical compounds. Biochem. Eng. J. 2018, 137, 182–191. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process. Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Sharma, T.; Sailwal, M.; Dasgupta, D.; Bhaskar, T.; Ghosh, D. Effect of lignocellulosic biomass inhibitors on oleaginous yeast cultivation in multistage fermentation system. Bioresour. Technol. Rep. 2021, 15, 100791–100796. [Google Scholar] [CrossRef]
- Tanimura, A.; Takashima, M.; Sugita, T.; Endoh, R.; Ohkuma, M.; Kishino, S.; Ogawa, J.; Shima, J. Lipid production through simultaneous utilization of glucose, xylose, and L-arabinose by Pseudozyma hubeiensis: A comparative screening study. AMB Express 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Qi, W.; Wang, Q.; Tan, X. The effect of metal salts on the decomposition of sweet sorghum bagasse in flow-through liquid hot water. Bioresour. Technol. 2011, 102, 3445–3450. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, M.; Liu, J.N.; Huang, X.F. Bioconversion of mixed volatile fatty acids into microbial lipids by Cryptococcus curvatus ATCC 20509. Bioresour. Technol. 2017, 241, 645–651. [Google Scholar] [CrossRef]
- Johnravindar, D.; Karthikeyan, O.P.; Selvam, A.; Murugesan, K.; Wong, J.W. Lipid accumulation potential of oleaginous yeasts: A comparative evaluation using food waste leachate as a substrate. Bioresour. Technol. 2018, 248, 221–228. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.; He, W.; He, T.; Lu, L.; Peng, K.; Huang, X. Integration of coagulation, acid separation and struvite precipitation as fermentation medium conditioning methods to enhance microbial lipid production from dewatered sludge. Bioresour. Technol. Rep. 2019, 7, 100221–100227. [Google Scholar] [CrossRef]
- Hofmeyer, T.; Hackenschmidt, S.; Nadler, F.; Thürmer, A.; Daniel, R.; Kabisch, J. Draft genome sequence of Cutaneotrichosporon curvatus DSM 101032 (formerly Cryptococcus curvatus), an oleaginous yeast producing polyunsaturated fatty acids. Genome Announc. 2016, 4, e00362-16. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.M.; Alper, H.S. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genet. Biol. 2016, 89, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Sutanto, S.; Zullaikah, S.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.-H. Lipomyces starkeyi: Its current status as a potential oil producer. Fuel Process. Technol. 2018, 177, 39–55. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Wang, Q.-M.; Theelen, B.; Groenewald, M.; Bai, F.-Y.; Boekhout, T. Phylogeny of tremellomycetous yeasts and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Stud. Mycol. 2015, 81, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Adrio, J.L. Oleaginous yeasts: Promising platforms for the production of oleochemicals and biofuels. Biotechnol. Bioeng. 2017, 114, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Kourist, R.; Bracharz, F.; Lorenzen, J.; Kracht, O.N.; Chovatia, M.; Daum, C.; Deshpande, S.; Lipzen, A.; Nolan, M.; Ohm, R.A. Genomics and transcriptomics analyses of the oil-accumulating basidiomycete yeast Trichosporon oleaginosus: Insights into substrate utilization and alternative evolutionary trajectories of fungal mating systems. MBio 2015, 6, e00918-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar]
- Bracharz, F.; Beukhout, T.; Mehlmer, N.; Brück, T. Opportunities and challenges in the development of Cutaneotrichosporon oleaginosus ATCC 20509 as a new cell factory for custom tailored microbial oils. Microb. Cell Factories 2017, 16, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Liu, L.; Zeng, A.P.; Wei, D. From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresour. Technol. 2017, 245, 1507–1519. [Google Scholar] [CrossRef]
- Gorte, O.; Aliyu, H.; Neumann, A.; Ochsenreither, K. Draft genome sequence of the oleaginous yeast Apiotrichum porosum (syn. Trichosporon porosum) DSM 27194. J. Genom. 2019, 7, 11–13. [Google Scholar]
- Schulze, I.; Hansen, S.; Großhans, S.; Rudszuck, T.; Ochsenreither, K.; Syldatk, C.; Neumann, A. Characterization of newly isolated oleaginous yeasts-Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express 2014, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.K.; Lorenzen, J.; Masri, M.; Liu, Y.; Baráth, E.; Brück, T.; Lercher, J.A. Catalytic decomposition of the oleaginous yeast Cutaneotrichosporon oleaginosus and subsequent biocatalytic conversion of liberated free fatty acids. ACS Sustain. Chem. Eng. 2019, 7, 6531–6540. [Google Scholar] [CrossRef]
- Ykema, A.; Verbree, E.; Van Verseveld, H.; Smit, H. Mathematical modelling of lipid production by oleaginous yeasts in continuous cultures. Antonie Leeuwenhoek 1986, 52, 491–506. [Google Scholar] [CrossRef]
- Ratledge, C. Single cell oils—Have they a biotechnological future? Trends Biotechnol. 1993, 11, 278–284. [Google Scholar] [CrossRef]
- Moreton, R. Yeast lipid estimation by enzymatic and nuclear magnetic resonance methods. Appl. Environ. Microbiol. 1989, 55, 3009–3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujjari, P.; Suh, S.O.; Coumes, K.; Zhou, J.J. Characterization of oleaginous yeasts revealed two novel species: Trichosporon cacaoliposimilis sp. nov. and Trichosporon oleaginosus sp. nov. Mycologia 2011, 103, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Takashima, M.; Ikeda, R.; Nakase, T.; Shinoda, T. Phylogenetic and taxonomic heterogeneity of Cryptococcus humicolus by analysis of the sequences of the internal transcribed spacer regions and 18S rDNA, and the phylogenetic relationships of C. humicolus, C. curvatus, and the genus Trichosporon. Microbiol. Immunol. 2000, 44, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Okoli, I.; Oyeka, C.A.; Kwon-Chung, K.J.; Theelen, B.; Robert, V.; Groenewald, J.Z.; McFadden, D.C.; Casadevall, A.; Boekhout, T. Cryptotrichosporon anacardii gen. nov., sp. nov., a new trichosporonoid capsulate basidiomycetous yeast from Nigeria that is able to form melanin on niger seed agar. FEMS Yeast Res. 2007, 7, 339–350. [Google Scholar] [CrossRef]
- Takashima, M.; Manabe, R.-I.; Iwasaki, W.; Ohyama, A.; Ohkuma, M.; Sugita, T. Selection of orthologous genes for construction of a highly resolved phylogenetic tree and clarification of the phylogeny of Trichosporonales species. PLoS ONE 2015, 10, e0138637. [Google Scholar] [CrossRef]
- Morrow, C.A.; Fraser, J.A. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res. 2009, 9, 161–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Z.; Hollinshead, W.; Isaguirre, C.; Tang, Y.J.; Liao, W.; Liu, Y. Effects of inhibitory compounds in lignocellulosic hydrolysates on Mortierella isabellina growth and carbon utilization. Bioresour. Technol. 2015, 183, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Casal, M.; Paiva, S.; Queirós, O.; Soares-Silva, I. Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 2008, 32, 974–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, M.; Sasano, Y.; Harashima, S. Mechanism of yeast adaptation to weak organic acid stress. In Stress Biology of Yeasts and Fungi, 1st ed.; Takagi, H., Kitagaki, H., Eds.; Springer: Berlin, Germany, 2015; pp. 107–121. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Improvement and modeling of culture parameters to enhance biomass and lipid production by the oleaginous yeast Cryptococcus curvatus grown on acetate. Bioresour. Technol. 2015, 192, 582–591. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 189. [Google Scholar] [CrossRef]
- Huang, X.; Chen, R.; Yuan, M.; Liu, J. Efficient bioconversion of high-content volatile fatty acids into microbial lipids by Cryptococcus curvatus ATCC 20509. Bioresour. Technol. 2017, 239, 394–401. [Google Scholar]
- Park, G.W.; Chang, H.N.; Jung, K.; Seo, C.; Kim, Y.C.; Choi, J.H.; Woo, H.C.; Hwang, I.J. Production of microbial lipid by Cryptococcus curvatus on rice straw hydrolysates. Process. Biochem. 2017, 56, 147–153. [Google Scholar] [CrossRef]
- Huang, X.F.; Shen, Y.; Luo, H.J.; Liu, J.N.; Liu, J. Enhancement of extracellular lipid production by oleaginous yeast through preculture and sequencing batch culture strategy with acetic acid. Bioresour. Technol. 2018, 247, 395–401. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; He, T.; Luo, H.; Peng, K.; Huang, X.; Liu, J. Application of de-lignified cellulose to enhance intracellular and extracellular lipid production from oleaginous yeast using acetic acid. Bioresour. Technol. 2019, 293, 122032–122039. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Validation of a predictive model for fed-batch and continuous lipids production processes from acetic acid using the oleaginous yeast Cryptococcus curvatus. Biochem. Eng. J. 2016, 111, 117–128. [Google Scholar] [CrossRef]
- Yuan, Q.; Sparling, R.; Oleszkiewicz, J. VFA generation from waste activated sludge: Effect of temperature and mixing. Chemosphere 2011, 82, 603–607. [Google Scholar] [CrossRef]
- Khiewwijit, R.; Temmink, H.; Labanda, A.; Rijnaarts, H.; Keesman, K.J. Production of volatile fatty acids from sewage organic matter by combined bioflocculation and alkaline fermentation. Bioresour. Technol. 2015, 197, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.N.; Kim, N.J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess. Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Vajpeyi, S.; Chandran, K. Microbial conversion of synthetic and food waste-derived volatile fatty acids to lipids. Bioresour. Technol. 2015, 188, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of crude glycerol from biodiesel plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Tyagi, R.D.; Drogui, P. Utilization of methanol in crude glycerol to assist lipid production in non-sterilized fermentation from Trichosporon oleaginosus. Bioresour. Technol. 2018, 253, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, W.; Shen, H.; Zhao, Z.K.; Yang, Z.; Yan, J.; Zhao, M. Co-utilization of corn stover hydrolysates and biodiesel-derived glycerol by Cryptococcus curvatus for lipid production. Bioresour. Technol. 2016, 219, 552–558. [Google Scholar] [CrossRef]
- Uprety, B.K.; Reddy, J.V.; Dalli, S.S.; Rakshit, S.K. Utilization of microbial oil obtained from crude glycerol for the production of polyol and its subsequent conversion to polyurethane foams. Bioresour. Technol. 2017, 235, 309–315. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P. Lipid production from fed-batch fermentation of crude glycerol directed by the kinetic study of batch fermentations. Fuel 2017, 209, 1–9. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.A.; Ignat, R.M. Enhanced methanol recovery and glycerol separation in biodiesel production–DWC makes it happen. Appl. Energy 2012, 99, 146–153. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Van Dijk, R.; Faber, K.N.; Kiel, J.A.; Veenhuis, M.; van der Klei, I. The methylotrophic yeast Hansenula polymorpha: A versatile cell factory. Enzyme Microb. Technol. 2000, 26, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Mayson, B.E.; Kilburn, D.G.; Zamost, B.L.; Raymond, C.K.; Lesnicki, G.J. Effects of methanol concentration on expression levels of recombinant protein in fed-batch cultures of Pichia methanolica. Biotechnol. Bioeng. 2003, 81, 291–298. [Google Scholar] [CrossRef]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Zhao, X.; Zhao, Z.K. Production of lipid from N-acetylglucosamine by Cryptococcus curvatus. Eur. J. Lipid Sci. Technol. 2010, 112, 727–733. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, W.; Liu, Y.; Yan, J.; Gong, Z. A two-stage process facilitating microbial lipid production from N-acetylglucosamine by Cryptococcus curvatus cultured under non-sterile conditions. Bioresour. Technol. 2018, 258, 255–262. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Wang, Q.; Yang, X.; Xie, H.; Zhao, Z.K. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol. Biofuels 2013, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.A.; Lavoie, J.M. From first-to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Deneyer, A.; Ennaert, T.; Sels, B.F. Straightforward sustainability assessment of sugar-derived molecules from first-generation biomass. Curr. Opin. Green Sustain. 2018, 10, 11–20. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.; de Boer, I.J.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Sec. 2020, 25, 100330–100340. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, J.R.; Wang, Y.T.; Zhu, M.J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756–122764. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, X.; Yuan, W.; Wang, Y.; Zhou, W.; Wang, G.; Liu, Y. Fed-batch enzymatic hydrolysis of alkaline organosolv-pretreated corn stover facilitating high concentrations and yields of fermentable sugars for microbial lipid production. Biotechnol. Biofuels 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chang, K.S.; Lee, C.F.; Hsu, C.L.; Huang, C.W.; Jang, H.D. Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy 2015, 72, 95–103. [Google Scholar] [CrossRef]
- Siebenhaller, S.; Kirchhoff, J.; Kirschhöfer, F.; Brenner-Weiß, G.; Muhle-Goll, C.; Luy, B.; Haitz, F.; Hahn, T.; Zibek, S.; Syldatk, C. Integrated process for the enzymatic production of fatty acid sugar esters completely based on lignocellulosic substrates. Front. Chem. 2018, 6, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mohan, S.V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour. Technol. 2018, 254, 284–289. [Google Scholar] [CrossRef]
- Samavi, M.; Uprety, B.K.; Rakshit, S. Bioconversion of poplar wood hemicellulose prehydrolysate to microbial oil using Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2019, 189, 626–637. [Google Scholar] [CrossRef]
- Brar, K.; Sarma, A.; Aslam, M.; Polikarpov, I.; Chadha, B. Potential of oleaginous yeast Trichosporon sp., for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour. Technol. 2017, 242, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Spanopoulos, A.; Matsakas, L. Single cell oil and ethanol production by the oleaginous yeast Trichosporon fermentans utilizing dried sweet sorghum stalks. Renew. Energy 2020, 146, 1609–1617. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, M.; Zou, T.; Peng, N.; Zhao, M.; Gong, Z. Phosphate removal combined with acetate supplementation enhances lipid production from water hyacinth by Cutaneotrichosporon oleaginosum. Biotechnol. Biofuels 2019, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, S.; Zhu, Z.; Shen, H.; Lin, X.; Jin, X.; Jiao, X.; Zhao, Z.K. Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol. Biofuels 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioelovich, M. Waste paper as promising feedstock for production of biofuel. J. Sci. Res. Rep. 2014, 3, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Choi, M.S.; Yang, J.K. Optimization of concentrated acid hydrolysis of waste paper using response surface methodology. J. Korean Wood Sci. Technol. 2013, 41, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sharifzadeh, M.; Templer, R.; Murphy, R.J. Bioethanol production from various waste papers: Economic feasibility and sensitivity analysis. Appl. Energy 2013, 111, 1172–1182. [Google Scholar] [CrossRef]
- Elliston, A.; Collins, S.R.; Wilson, D.R.; Roberts, I.N.; Waldron, K.W. High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste-paper. Bioresour. Technol. 2013, 134, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Gong, Z.; Zhang, L.; Liu, Y.; Yan, J.; Zhao, M. Feasibility of lipid production from waste paper by the oleaginous yeast Cryptococcus curvatus. Bioresources 2017, 12, 5249–5263. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Infante, E.G.; Secchi, A.R.; Leite, L.F.; de Souza Antunes, A.M. Scientific Articles and Patent Applications on Biodiesel Production from Lignocellulosic Biomass. J. Environ. Prot. Sci. 2021, 12, 371–390. [Google Scholar] [CrossRef]
- Sijtsma, L.; De Swaaf, M. Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004, 64, 146–153. [Google Scholar] [CrossRef]
- Somacal, S.; Pinto, V.S.; Vendruscolo, R.G.; Somacal, S.; Wagner, R.; Ballus, C.A.; Kuhn, R.C.; Mazutti, M.A.; Menezes, C.R. Maximization of microbial oil containing polyunsaturated fatty acid production by Umbelopsis (Mortierella) isabellina. Biocatal. Agric. Biotechnol. 2020, 30, 101831–101840. [Google Scholar] [CrossRef]
- Diwan, B.; Gupta, P. Synthesis of MCFA and PUFA rich oils by enzymatic structuring of flax oil with single cell oils. LWT 2020, 133, 109928–109934. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Yaguchi, A.; Blenner, M. Oleaginous yeast for biofuel and oleochemical production. Curr. Opin. Biotechnol. 2019, 57, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Steiger, M.G.; Rassinger, A.; Mattanovich, D.; Sauer, M. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metab. Eng. 2019, 52, 224–231. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, S.; Bilal, M.; Ge, X.; Zhang, C.; Fickers, P.; Cheng, H. Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb. Cell Fact. 2018, 17, 133. [Google Scholar] [CrossRef]
- Janek, T.; Dobrowolski, A.; Biegalska, A.; Mirończuk, A.M. Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb. Cell Fact. 2017, 16, 118. [Google Scholar] [CrossRef] [Green Version]
- Bhutada, G.; Kavšček, M.; Ledesma-Amaro, R.; Thomas, S.; Rechberger, G.N.; Nicaud, J.M.; Natter, K. Sugar versus fat: Elimination of glycogen storage improves lipid accumulation in Yarrowia lipolytica. FEMS Yeast Res. 2017, 17, 20–29. [Google Scholar] [CrossRef]
- Beopoulos, A.; Mrozova, Z.; Thevenieau, F.; Le Dall, M.T.; Hapala, I.; Papanikolaou, S.; Chardot, T.; Nicaud, J.M. Control of lipid accumulation in the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2008, 74, 7779–7789. [Google Scholar] [CrossRef] [Green Version]
- Görner, C.; Redai, V.; Bracharz, F.; Schrepfer, P.; Garbe, D.; Brück, T. Genetic engineering and production of modified fatty acids by the non-conventional oleaginous yeast Trichosporon oleaginosus ATCC 20509. Green Chem. 2016, 18, 2037–2046. [Google Scholar] [CrossRef] [Green Version]
- Koivuranta, K.; Castillo, S.; Jouhten, P.; Ruohonen, L.; Penttilä, M.; Wiebe, M.G. Enhanced triacylglycerol production with genetically modified Trichosporon oleaginosus. Front. Microbiol. 2018, 9, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, G.Y.; Han, J.I. Biodiesel production from oleaginous yeast, Cryptococcus sp. by using banana peel as carbon source. Energy Rep. 2019, 5, 1077–1081. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, T.; Nautiyal, A.K.; Dasgupta, D.; Hazra, S.; Bhaskar, T.; Ghosh, D. Scale-up strategy for yeast single cell oil production for Rhodotorula mucilagenosa IIPL32 from corn cob derived pentosan. Bioresour. Technol. 2020, 309, 123329–123337. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of algae and yeast-derived single cell oils: A critical review of their role in improving biorefinery sustainability. Bioresour. Technol. 2020, 303, 122862–122872. [Google Scholar] [CrossRef]
- Shen, Q.; Lin, H.; Wang, Q.; Fan, X.; Yang, Y.; Zhao, Y. Sweetpotato vines hydrolysate promotes single cell oils production of Trichosporon fermentans in high-density molasses fermentation. Bioresour. Technol. 2015, 176, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production strategies and applications of microbial single cell oils. Front. Microbiol. 2016, 7, 1539–1564. [Google Scholar] [CrossRef] [Green Version]
- Abril, J.R.; Wills, T.; Harding, F. Applications of single cell oils for animal nutrition. In Single Cell Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; Elsevier: Urbana, IL, USA, 2010; Volume 18, pp. 389–419. [Google Scholar]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of single-cell ingredients in aquaculture feeds—A review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current pretreatment/cell disruption and extraction methods used to improve intracellular lipid recovery from oleaginous yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [Green Version]
- Imatoukene, N.; Koubaa, M.; Perdrix, E.; Benali, M.; Vorobiev, E. Combination of cell disruption technologies for lipid recovery from dry and wet biomass of Yarrowia lipolytica and using green solvents. Process. Biochem. 2020, 90, 139–147. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Yook, S.; Kim, J.; Woo, H.M.; Um, Y.; Lee, S.M. Efficient lipid extraction from the oleaginous yeast Yarrowia lipolytica using switchable solvents. Renew. Energy 2019, 132, 61–67. [Google Scholar] [CrossRef]

| AA (g/L) | FT | C/N (g/g) | pH | T (°C) | CX (g/L) | CL (g/L) | YLX (w/w%) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n.a. | F | 10 | 6 | 30 | 1.7 | 0.3 | 15 | 6 | 17.5 | 20.5 | 49.1 | [60] |
| F | 10 | 7 | 30 | 2.2 | 0.4 | 18 | 9 | 19.3 | 19 | 47.5 | ||
| F | 10 | 7 | 30 | 80 | 12 | 15 | 15 | 20.2 | 17.6 | 44.6 | ||
| n.a. | B | 50 | 7 | 30 | 8.1 | 4.2 | 49.9 | 15 | 32 | 23.6 | 39.5 | [61] |
| 30 | B | 100 | 7 | 30 | 7.2 | 4.2 | 58 | 40.4 | 17.5 | 15.8 | 29.5 | [31] |
| 30 | B | 62.5 | 9 | 30 | 8.4 | 4.9 | 58.3 | 17.2 | n.a. | n.a. | n.a. | [62] |
| 40 | B | 62.5 | 9 | 30 | 9.7 | 6.1 | 62.5 | 15.9 | n.a. | n.a. | n.a. | |
| 6 | F | 40 | 5.5 | 25 | 3.2 | 1 | 32.2 | 17 | 12.3 | 17.4 | 50.3 | [63] |
| 5 | B | 10 | 7 | 30 | 2.1 | 0.5 | 24 | 30 | 9.4 | 19.4 | 51.6 | [64] |
| 10 | B | 10 | 7 | 30 | 3.4 | 1.2 | 35.6 | 30 | 9 | 20.4 | 55.2 | |
| 20 | B | 10 | 7 | 30 | 4.8 | 2.9 | 60.2 | 35 | 8 | 22.5 | 55.3 | |
| 30 | B | 10 | 7 | 30 | 6.3 | 3.7 | 58.9 | 30 | 7.8 | 22.2 | 54 | |
| 40 | B | 10 | 7 | 30 | 7 | 5 | 71.7 | 32 | 8.4 | 29.8 | 50.4 | |
| 30 | B | 59 | 8 | 34.7 | 6.7 | 5.5 | 82.1 | 25 | 7 | 31.2 | 44.8 | [65] |
| 30 | B a | 60.5 | 8 | 30.8 | 7.9 | 6.2 | 78.3 | 23 | 6.5 | 28.8 | 49.7 | |
| 30 | B b | 59.4 | 8 | 29.4 | 8.8 | 5.2 | 59.1 | 19 | 9.5 | 27.3 | 49.4 | |
| 40 | B | 58.7 | 8 | 36.6 | 6.2 | 3.8 | 61.1 | 18 | 5.6 | 32.6 | 41.4 | |
| 40 | B a | 59.2 | 8 | 39 | 6.3 | 4.9 | 77.8 | 17 | 6 | 34.1 | 42.3 | |
| 40 | B b | 58.7 | 8 | 27.5 | 12.8 | 7.2 | 56.3 | 20 | 8.9 | 26.2 | 50.3 |
| VOAs (g/L) | FT | C/N (g/g) | CX (g/L) | CL (g/L) | YLX (w/w%) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 a | F | 10 | 7.7 | 1.1 | 14 | 5 | 19 | 25.3 | 47.7 | [68] |
| 5 a | C | 50 | 26.7 | 13.6 | 51 | 13.4 | 13.5 | 26.9 | 51.4 | |
| 18:9:3 b | B | 62.5 | 9 | 4.8 | 53.3 | 18.7 | n.a. | n.a. | n.a. | [62] |
| 24:12:4 b | B | 62.5 | 11.8 | 7.5 | 63.2 | 18.7 | n.a. | n.a. | n.a. | |
| 15:0:15 b | B | 100 | 8.3 | 4.8 | 57.1 | 41.4 | 16.3 | 22.4 | 35.3 | [31] |
| 15:5:10 b | B | 100 | 8.7 | 4.9 | 56.9 | 37.6 | 12.7 | 14.1 | 31.6 | |
| 15:10:5 b | B | 100 | 8 | 4.6 | 57.2 | 33.4 | 11.1 | 9.9 | 33.2 | |
| 15:15:0 b | B | 100 | 7.6 | 4 | 52.1 | 27.1 | 9.8 | 9.4 | 30 | |
| 10:5:15 b | B | 100 | 8.4 | 4.7 | 56.5 | 33.2 | 11 | 8 | 31.5 | |
| 10:10:10 b | B | 100 | 8.1 | 4.3 | 52.5 | 31.2 | 9.7 | 7.9 | 30.7 | |
| 10:15:5 b | B | 100 | 6.9 | 3.3 | 48.2 | 25.6 | 9.2 | 8.1 | 29.6 | |
| 5:10:15 b | B | 100 | 8.2 | 4 | 48.4 | 28.1 | 9.1 | 5.2 | 27.3 | |
| 5:15:10 b | B | 100 | 8.2 | 3.8 | 46.5 | 27.3 | 8.8 | 3.5 | 26.1 | |
| 0:15:15 b | B | 100 | 7.4 | 2.4 | 32.4 | 16.7 | 9.3 | 3.4 | 26.4 |
| Carbon Source (g/L) | FT | C/N (g/g) | pH | CX (g/L) | CL (g/L) | YLX (w/w%) | YLT (g/L/h) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG 10.3 | B | 20 | 5.5 | 13.4 | 3.1 | 23 | 0.05 | n.a. | n.a. | n.a. | n.a. | [78] |
| CG 15.3 | B | 30 | 5.5 | 23.7 | 11.3 | 47.5 | 0.21 | n.a. | n.a. | n.a. | n.a. | |
| CG 22.8 | B | 45 | 5.5 | 24.8 | 12.1 | 49 | 0.22 | n.a. | n.a. | n.a. | n.a. | |
| CG 29.7 | B | 60 | 5.5 | 19.3 | 10 | 52 | 0.18 | n.a. | n.a. | n.a. | n.a. | |
| CG 46.3 | F | 45 | 5.5 | 43.8 | 21.9 | 49.9 | 0.42 | n.a. | n.a. | n.a. | n.a. | |
| CG 46.3 | F a | 45 | 5 | 43.2 | 20.8 | 48.1 | 0.35 | n.a. | 30 | n.a. | 46 | [75] |
| CG 20.0 | B | 30 | 7 | 5.6 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | [79] |
| CG 40.0 | B | 30 | 7 | 2.2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| CG 60.0 | B | 30 | 7 | 0.4 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| CG 80.0 | B | 30 | 7 | 0.5 | n.a. | n.a | n.a. | n.a. | n.a. | n.a. | n.a. | |
| CG 25.8 | F | 30 | 5.5 | 31.2 | n.a. | 44.6 | 1.2 | n.a | 23 | 16.7 | 39.6 | |
| CG 32.0 | F | 30 | 5.5 | 32.9 | n.a. | 52.9 | 1.5 | n.a. | n.a. | n.a. | n.a. | |
| Glu 40 + Xyl 20 | B | 27 | 5.5 | 25.5 | 10.4 | 40.7 | 0.14 | 16.5 | 29 | 12.5 | 46.8 | [76] |
| Glu 40 + Xyl 20 + CG 30 | B | 27 | 5.5 | 34.4 | 16.8 | 48.7 | 0.17 | 18.3 | 27.2 | 12.7 | 49.8 | |
| Xyl 30 + CG 30 | B | 27 | 5.5 | 25.8 | 10 | 38.8 | 0.12 | 16.3 | 28.7 | 13.7 | 45.2 | |
| CG 30 | B | 27 | 5.5 | 15.4 | 4.2 | 27.3 | 0.09 | 13.6 | 23.3 | 18 | 48.4 | |
| CSEH | B | 27 | 5.5 | 11.8 | 4.6 | 39.4 | 0.08 | 15.9 | 29.6 | 13.4 | 47.2 | |
| CSEH + CG 30 | B | 27 | 5.5 | 21.7 | 10.8 | 49.7 | 0.13 | 18 | 34.2 | 13.4 | 46.3 |
| N-Acetylglucosamine (g/L) | FT | C/N (g/g) | T (°C) | CX (g/L) | CL (g/L) | YLX (w/w%) | YLT (g/L/h) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 | B | 7.2 | 22 | 17.6 | 8.8 | 50 | 0.07 | 17.1 | 31.3 | 21.9 | 41.6 | [85] |
| 70 | B | 7.2 | 26 | 16.9 | 9.1 | 54 | 0.08 | 16 | 24 | 18.2 | 47.4 | |
| 70 | B | 7.2 | 30 | 19.1 | 8.6 | 45 | 0.07 | 15.5 | 23.9 | 19 | 51.1 | |
| 70 | B | 7.2 | 35 | 13.8 | 7.2 | 52 | 0.06 | 14.2 | 24.2 | 19.5 | 51.6 | |
| 90 | B | 7.2 | 26 | 22.9 | 10.1 | 44 | 0.08 | 15.3 | n.a. | n.a. | n.a. | |
| 110 | B | 7.2 | 26 | 20.3 | 9.7 | 48 | 0.08 | 16.2 | n.a. | n.a. | n.a. | |
| 40 | B | 8 | 30 | 16.8 | 5.1 | 30.5 | 0.11 | 15 | 40.2 | 9.5 | 42.3 | [86] |
| 40 | B a | 8 | 30 | 16.2 | 8.4 | 51.6 | 0.12 | 22 | n.a. | n.a. | n.a. | |
| 40 | B a,b | 8 | 30 | 17.4 | 9.9 | 56.9 | 0.17 | 23 | 43.6 | 9.8 | 40.1 |
| Carbon Source (g/L) | FT | C/N (g/g) | pH | T (°C) | CX (g/L) | CL (g/L) | YLX (w/w%) | YLT (g/L/h) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardoon stalks (Glu 90.0 + Xyl 9.4) | B a,1 | 85 | 5.5 | 30 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | [7] |
| B a,2 | 85 | 5.5 | 30 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| B b,1 | 85 | 5.5 | 30 | 26 | 6.8 | 26.2 | 0.02 | 9.9 | 22.6 | 10 | 39.5 | ||
| B b,2 | 85 | 5.5 | 30 | 22.8 | 7.5 | 32.9 | 0.02 | 13.6 | 21.6 | 11.5 | 37.8 | ||
| Corncob (Glu 40 + Xyl n.a.) | B | 60 | 6 | 25 | 8.2 | 4.7 | 59.1 | 0.05 | 12 | 21.1 | 7.1 | 58.9 | [94] |
| Corncob (Glu 60 + Xyl n.a.) | B | 90 | 6 | 25 | 12.6 | 7.6 | 60.2 | 0.08 | 13 | 21 | 7.5 | 57.5 | |
| Beech wood (Glu 41.7 + Xyl 6.2 + Cell 2.1) | F | 10 | 5 | 28 | 44.9 | 21.9 | 47.9 | 0.23 | 10 | 35.1 | 12.6 | 44.6 | [95] |
| Vegetable waste (TRS 472.4) c | B | 45 | 6.5 | 28 | 9.5 | 2.7 | 28.3 | 0.01 | n.a. | 26.5 | 9.2 | 53.2 | [96] |
| Vegetable waste (TRS 439.1) d | B | 45 | 6.5 | 28 | 8.1 | 2.1 | 26 | 0.01 | n.a. | 25.3 | 10.6 | 57.7 | |
| Poplar wood (Glu 7.1 + Xyl 143.9) | B | 200 | 5.5 | 30 | 13.9 | 5.1 | 37 | 0.05 | 19 | 15.7 | 9 | 45.9 | [97] |
| Giant reed (Glu 90.1 + Xyl 20.8) | F | 24 | 5.5 | 30 | 44.7 | 28.4 | 63.6 | 0.09 | 19.8 | 26.9 | 8.2 | 33.3 | [17] |
| Sugarcane bagasse (TRS 80.0) | B | n.a. | 5 | 30 | 25.3 | 10.3 | 40.5 | 0.09 | 14 | 22.9 | 10.4 | 54.7 | [98] |
| Sweet sorghum stalks (n.a.) | B | 60 | 6.5 | 30 | n.a. | 2 | n.a. | 0.02 | 6.7 | 23 | 4.6 | 37.9 | [99] |
| Water hyacinth (TRS 35.0) | B | 0.5 | 7 | 30 | 12.7 | 1.4 | 10.7 | 0.02 | 5.6 | n.a. | n.a. | n.a. | [100] |
| B e | 12.3 | 7 | 30 | 12.4 | 4.5 | 35.8 | 0.05 | 17.9 | n.a. | n.a. | n.a. | ||
| B f | 13.6 | 7 | 30 | 11.4 | 3.6 | 31.4 | 0.04 | 9.2 | n.a. | n.a. | n.a. | ||
| B e,f | 13.6 | 7 | 30 | 12.2 | 7.3 | 59.7 | 0.09 | 19.6 | 48.4 | 3 | 43 | ||
| Corn stover (TRS 70.0) | F | n.a. | 6 | 30 | 50.7 | 31.3 | 61.7 | 0.12 | 18 | 27.5 | 12 | 49 | [93] |
| Carbon Source | FT | C/N (g/g) | pH | T (°C) | CX (g/L) | CL (g/L) | YLX (w/w%) | YLT (g/L/h) | YLS (w/w%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPEH | B | 45.9 | 5.5 | 30 | 17.3 | 9.1 | 52.5 | 0.19 | 20.1 | 32.9 | 7.6 | 52.3 | [106] |
| NPEH | B | 47.2 | 5.5 | 30 | 14.7 | 7.5 | 51.4 | 0.16 | 20.9 | 32.6 | 6.8 | 51.5 | |
| CBEH | B | 53.7 | 5.5 | 30 | 12.5 | 7.1 | 56.4 | 0.15 | 22.4 | 30.2 | 6 | 55.2 | |
| WOP-H2O2 | B | 80 | 6 | 30 | 15.2 | 5.8 | 37.8 | 0.08 | 23.5 | 21.6 | 12.4 | 52.3 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fidio, N.; Minonne, F.; Antonetti, C.; Raspolli Galletti, A.M. Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes. Catalysts 2021, 11, 1291. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111291
Di Fidio N, Minonne F, Antonetti C, Raspolli Galletti AM. Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes. Catalysts. 2021; 11(11):1291. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111291
Chicago/Turabian StyleDi Fidio, Nicola, Filippo Minonne, Claudia Antonetti, and Anna Maria Raspolli Galletti. 2021. "Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes" Catalysts 11, no. 11: 1291. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111291