Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties
Abstract
:1. Introduction
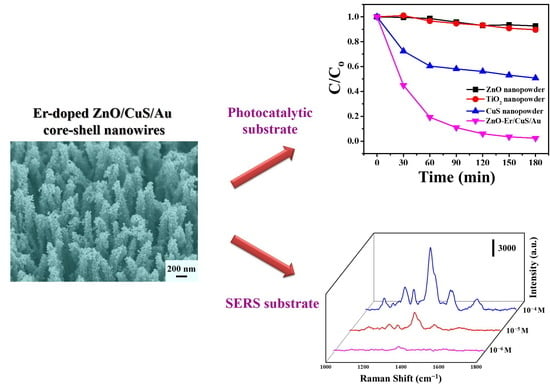
2. Results and Discussion
3. Material and Methods
3.1. Preparation of Er-Doped ZnO Nanowires
3.2. Preparation of Er-Doped ZnO/CuS/Au Core-Shell Nanowires
3.3. Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-Schematic Water Splitting into H2 and O2 Using Metal Sulfide as a Hydrogen-Evolving Photocatalyst and Reduced Graphene Oxide as a Solid-State Electron Mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hakari, Y.; Ikeda, S.; Jia, Q.; Iwase, A.; Kudo, A. Utilization of Metal Sulfide Material of (CuGa)1–xZn2xS2 Solid Solution with Visible Light Response in Photocatalytic and Photoelectrochemical Solar Water Splitting Systems. J. Phys. Chem. Lett. 2015, 6, 1042–1047. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.-J. Recent Progress on Metal Sulfide Composite Nanomaterials for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Malankowska, A.; Kulesza, D.; Sowik, J.; Cavdar, O.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite. Catalysts 2020, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci.-Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Li, F.; Wu, J.; Qin, Q.; Li, Z.; Huang, X. Controllable synthesis, optical and photocatalytic properties of CuS nanomaterials with hierarchical structures. Powder Technol. 2010, 198, 267–274. [Google Scholar] [CrossRef]
- Hai, Z.; Huang, J.; Remita, H.; Chen, J. Radiolytic synthesis of CuS nanotubes with photocatalytic activity under visible light. Mater. Lett. 2013, 108, 304–307. [Google Scholar] [CrossRef]
- Son, N.; Heo, J.N.; Youn, Y.-S.; Kim, Y.; Do, J.Y.; Kang, M. Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts. Catalysts 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.-L.; Chen, H.; Zhang, Y.C.; Le Wu, C. CuS nanostructures prepared by a hydrothermal method. J. Alloys Compd. 2011, 509, 6382–6387. [Google Scholar] [CrossRef]
- Wang, F.; Dong, H.; Pan, J.; Li, J.; Li, Q.; Xu, D. One-Step Electrochemical Deposition of Hierarchical CuS Nanostructures on Conductive Substrates as Robust, High-Performance Counter Electrodes for Quantum-Dot-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 19589–19598. [Google Scholar] [CrossRef]
- Hong, X.; Xu, Z.; Zhang, F.; He, C.; Gao, X.; Liu, Q.; Guo, W.; Liu, X.; Ye, M. Sputtered seed-assisted growth of CuS nanosheet arrays as effective counter electrodes for quantum dot-sensitized solar cells. Mater. Lett. 2017, 203, 73–76. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velmurugan, S.; Yang, T.C.K. One-pot sonochemical synthesis of CuS nanoplates decorated partially reduced graphene oxide for biosensing of dopamine neurotransmitter. Ultrason. Sonochem. 2020, 64, 105043. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Zhang, C.; Zhu, H. 3D Flowerlike Copper Sulfide Nanostructures Synthesized from Copper (I) Oxide Hollow Microspheres. Procedia Eng. 2012, 36, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Qian, X.; Ma, X.; Gong, Q.; Cao, H.; Yin, J. Shape-Controlled Synthesis and Self-Assembly of Hexagonal Covellite (CuS) Nanoplatelets. Chem. Eur. J. 2007, 13, 3241–3247. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Deng, C.; Niu, C.; Le, H. Microwave-assisted controllable synthesis of hierarchical CuS nanospheres displaying fast and efficient photocatalytic activities. J. Mater. Sci. 2018, 53, 14250–14261. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Yu, S.; Zhu, W.; Han, C.; Cao, X. Preparation and electrochemical performance of hierarchical CuS-rGO composite. J. Alloys Compd. 2017, 694, 1067–1072. [Google Scholar] [CrossRef]
- Tailor, J.P.; Chaki, S.H.; Deshpande, M.P. Comparative study between pure and manganese doped copper sulphide (CuS) nanoparticles. Nano Express 2021, 2, 010011. [Google Scholar] [CrossRef]
- Nath, S.K.; Kalita, P.K. Temperature dependent structural, optical and electrical properties of CuS nanorods in aloe vera matrix. Nano-Struct. Nano-Objects 2021, 25, 100651. [Google Scholar] [CrossRef]
- Castillón-Barraza, F.F.; Farías, M.H.; Coronado-López, J.H.; Encinas-Romero, M.A.; Pérez-Tello, M.; Herrera-Urbina, R.; Posada-Amarillas, A. Synthesis and Characterization of Copper Sulfide Nanoparticles Obtained by the Polyol Method. Adv. Sci. Lett. 2011, 4, 596–601. [Google Scholar] [CrossRef]
- Mao, J.; Shu, Q.; Wen, Y.; Yuan, H.; Xiao, D.; Choi, M.M.F. Facile Fabrication of Porous CuS Nanotubes Using Well-Aligned [Cu(tu)]Cl·1/2H2O Nanowire Precursors as Self-Sacrificial Templates. Cryst. Growth Des. 2009, 9, 2546–2548. [Google Scholar] [CrossRef]
- Pandey, S.; Fosso-Kankeu, E.; Redelinghuys, J.; Kim, J.; Kang, M. Implication of biofilms in the sustainability of acid mine drainage and metal dispersion near coal tailings. Sci. Total Environ. 2021, 788, 147851. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Goswami, G.K.; Okoro, H.K.; Fosso-Kankeu, E. Carbon Nanotubes in the 21st Century: An Advancement in Real Time Monitoring and Control of Environmental Water. In Nano and Bio-Based Technologies for Wastewater Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 265–301. [Google Scholar]
- Chen, J.; Xiong, Y.; Duan, M.; Li, X.; Li, J.; Fang, S.; Qin, S.; Zhang, R. Insight into the Synergistic Effect of Adsorption–Photocatalysis for the Removal of Organic Dye Pollutants by Cr-Doped ZnO. Langmuir 2020, 36, 520–533. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Asiri, A.M. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int. J. Biol. Macromol. 2015, 81, 584–590. [Google Scholar] [CrossRef]
- Thuy, U.T.D.; Liem, N.Q.; Parlett, C.M.A.; Lalev, G.M.; Wilson, K. Synthesis of CuS and CuS/ZnS core/shell nanocrystals for photocatalytic degradation of dyes under visible light. Catal. Commun. 2014, 44, 62–67. [Google Scholar] [CrossRef]
- Qin, N.; Wei, W.; Huang, C.; Mi, L. An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes. Catalysts 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Garg, N.; Ganguli, A.K. A type-II semiconductor (ZnO/CuS heterostructure) for visible light photocatalysis. J. Mater. Chem. A 2014, 2, 7517–7525. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Chen, S.; Zhang, K.; Zhang, J.; Zou, W.; Wang, R. One-step fabrication of BiOCl/CuS heterojunction photocatalysts with enhanced visible-light responsive activity. Mater. Chem. Phys. 2015, 158, 67–73. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ravi, P.; Sathish, M. Cauliflower-like CuS/ZnS nanocomposites decorated g-C3N4 nanosheets as noble metal-free photocatalyst for superior photocatalytic water splitting. Chem. Eng. J. 2019, 360, 1277–1286. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Photocatalytic hydrogen production with CuS/ZnO from aqueous Na2S + Na2SO3 solution. Int. J. Hydrogen Energy 2013, 38, 8625–8630. [Google Scholar] [CrossRef]
- Wang, Q.; An, N.; Bai, Y.; Hang, H.; Li, J.; Lu, X.; Liu, Y.; Wang, F.; Li, Z.; Lei, Z. High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2. Int. J. Hydrogen Energy 2013, 38, 10739–10745. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Feng, Y.; Li, Z. Efficient photocatalytic performance enhancement in Co-doped ZnO nanowires coupled with CuS nanoparticles. Appl. Surf. Sci. 2018, 428, 154–164. [Google Scholar] [CrossRef]
- Kumar Sonwani, R.; Pandey, S.; Kumar Yadav, S.; Shekhar Giri, B.; Katiyar, V.; Sharan Singh, R.; Nath Rai, B. Construction of integrated system for the treatment of Acid orange 7 dye from wastewater: Optimization and growth kinetic study. Bioresour. Technol. 2021, 337, 125478. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y.; Chen, C.-M. Double-sided plasmonic Au nanoparticles on Cu-doped ZnO/ZnO heterostructures with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 60–63. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Barman, D.; De, S.K. Maximization of photocatalytic activity of Bi2S3/TiO2/Au ternary heterostructures by proper epitaxy formation and plasmonic sensitization. Appl. Catal. B 2017, 219, 287–300. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y. Double-sided plasmonic silver nanoparticles decorated copper oxide/zinc oxide heterostructured nanomaces with improving photocatalytic performance. J. Photochem. Photobiol. A Chem. 2019, 378, 184–191. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, J.-C.; Chou, C.-M. H2Ti3O7 nanowires as a high-performance photocatalytic and surface-enhanced Raman scattering substrate. J. Photochem. Photobiol. A Chem. 2020, 400, 112666. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Wu, S.-H. Bi-functional Al-doped ZnO@SnO2 heteronanowires as efficient substrates for improving photocatalytic and SERS performance. J. Indust. Eng. Chem. 2019, 76, 333–343. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Barnes, A.J.; Majid, M.A.; Stuckey, M.A.; Gregory, P.; Stead, C.V. The resonance Raman spectra of Orange II and Para Red: Molecular structure and vibrational assignment. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 1985, 41, 629–635. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-M.; Chang, T.-T.; Chen, C.-Y.; Chang, Y.-C. Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties. Catalysts 2021, 11, 1347. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111347
Chou C-M, Chang T-T, Chen C-Y, Chang Y-C. Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties. Catalysts. 2021; 11(11):1347. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111347
Chicago/Turabian StyleChou, Chia-Man, Tan-Tzu Chang, Chin-Yi Chen, and Yu-Cheng Chang. 2021. "Constructing Er-Doped ZnO/CuS/Au Core-Shell Nanowires with Enhanced Photocatalytic and SERS Properties" Catalysts 11, no. 11: 1347. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111347