α-Diimine Ni-Catalyzed Ethylene Polymerizations: On the Role of Nickel(I) Intermediates
Abstract
:1. Introduction
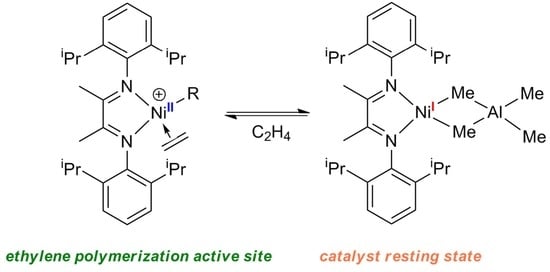
2. Cationic Ni(II) α-Diimine Complexes in Ethylene Polymerization
3. Ni(I) α-Diimine Complexes in Ethylene Polymerization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.L.; Liang, G.D.; Gao, H.Y.; Wu, Q. Enhancing thermal stability and living fashion in α-diimine-nickel catalyzed (co)polymerization of ethylene and polar monomers by increasing the steric bulk of ligand backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.B.; Solan, G.A.; Sun, W.-H. Recent advances in Niimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α-diimine catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Lian, K.B.; Kong, W.Y.; Xu, S.; Jiang, G.R.; Dai, S.Y. Synthesis of various branched ultra-high-molecular-weight polyethylenes using sterically hindered acenaphthene-based α-diimine Ni(II) catalysts. Organometallics 2018, 37, 2442–2449. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.L.; Li, Y.G.; Chen, C.L. Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 30, 4143–4149. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C.L. Emerging palladium and nickel catalysts for copolymerization of olefins with polar monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.D.; Zhang, Y.X.; Cui, L.; Mu, H.L.; Jian, Z.B. Pentiptycenyl substituents in insertion polymerization with α-diimine nickel and palladium species. Organometallics 2019, 38, 2075–2083. [Google Scholar] [CrossRef]
- Gong, Y.F.; Li, S.K.; Gong, Q.; Zhang, S.J.; Liu, B.Y.; Dai, S.Y. Systematic investigations of ligand steric effects on α-diimine nickel catalyzed olefin polymerization and copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Bryliakov, K.P.; Antonov, A.A.; Talsi, E.P. Ethylene polymerization of nickel catalysts with α-diimine ligands: Factors controlling the structure of active species and polymer properties. Dalton Trans. 2019, 48, 7974–7984. [Google Scholar] [CrossRef]
- Liang, T.; Goundari, S.B.; Chen, C. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Zhang, Y.X.; Kou, S.Q.; Jian, Z.B. A concerted double-layer steric strategy enables an ultra-highly active nickel catalysts to access ultrahigh molecular weight polythylens. J. Catal. 2020, 90, 30–36. [Google Scholar] [CrossRef]
- Tran, Q.H.; Brookhart, M.; Daugulis, O. New neutral nickel and palladium sandwich catalysts: Synthesis of ultrahigh molecular weight polyethylene (UHMWPE) via highly controlled polymerization and mechanistic studies of chain propagation. J. Am. Chem. Soc. 2020, 142, 7198–7206. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Wang, C.Q.; Jian, Z.B. Comprehensive studies of ligand electronic effect on unsymmetrical α-diimine nickel(II) promoted ethylene (co)polymerizations. Polym. Chem. 2020, 11, 4005–4012. [Google Scholar] [CrossRef]
- Li, S.K.; Xu, S.Y.; Dai, S.Y. A remote nonconjugated electron effect in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 2692–2699. [Google Scholar] [CrossRef]
- Muhammad, Q.; Tan, C.; Chen, C.L. Concerted steric and electronic effects on α-diimine nickel- and palladium-catalyzed ethylene polymerization and copolymerization. Sci. Bull. 2020, 65, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.Q.; Zhang, Y.X.; Jian, Z.B. Unsymmetrical strategy makes significant differences in α-diimine nickel and palladium catalyzed ethylene copolymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Dai, S.Y.; Li, S.K.; Xu, G.Y.; Wu, C.; Liao, Y.D.; Guo, L.H. Flexible cycloalkyl substituents in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 1393–1400. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polymer J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Svejda, S.A.; Johnson, L.K.; Brookhart, M. Low-Temperature Spectroscopic Observation of Chain Growth and Migratory Insertion Barriers in (α-Diimine)Ni(II) Olefin Polymerization Catalysts. J. Am. Chem. Soc. 1999, 121, 10634–10635. [Google Scholar] [CrossRef]
- Antonov, A.A.; Samsonenko, D.G.; Talsi, E.P.; Bryliakov, K.P. Formation of Cationic Intermediates upon the Activation of Bis(imino)pyridine Nickel Catalysts. Organometallics 2013, 32, 2187–2191. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Zakharov, V.A.; Moller, H.M.; Olscher, F.; Osichow, A.; Gottker-Schnettmann, I.; Mecking, S.; Talsi, E.P.; Bryliakov, K.P. Formation and Evolution of Chain-Propagating Species Upon Ethylene Polymerization with Neutral Salicylaldiminato Nickel(II) Catalysts. Chem. Eur. J. 2013, 19, 11409–11417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Zakharov, V.A.; Talsi, E.P. NMR spectroscopic identification of Ni(II) species formed upon activation of (α-diimine)NiBr2 polymerization catalysts with MAO and MMAO. Dalton Trans. 2018, 47, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. Nature of Heterobinuclear Ni(I) Complexes Formed upon the Activation of the α-Diimine Complex LNiIIBr2 with AlMe3 and MMAO. Organometallics 2021, 40, 907–914. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. Activation of an α-diimine Ni(II) precatalysts with AlMe3 and Al(iBu)3: Catalytic and NMR and EPR spectroscopic studies. Organometallics 2020, 39, 3034–3040. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. EPR spectroscopic study of Ni(I) species in the catalyst system for ethylene polymerization based on α-diimine Ni(II) complex activated by MMAO. J. Organomet. Chem. 2019, 880, 267–271. [Google Scholar] [CrossRef]
- Gurinovich, N.S.; Petrovsky, S.K.; Saliy, I.V.; Saraev, V.V. Influence of a diimine ligand and an activator on the processes taking place in Brookhart-type nickel catalytic systems. Res. Chem. Intermed. 2018, 44, 1935–1944. [Google Scholar] [CrossRef]
- Gurinovich, N.S.; Petrovskii, S.K.; Saraev, V.V.; Salii, I.V. Study of the Nature and Mechanism of the Formation of Paramagnetic Species in Nickel-Based Brookhart-Type Catalytic Systems. Kinet. Catal. 2016, 57, 523–527. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Belykh, L.B.; Schmidt, F.K. EPR Spectroscopy of catalytic systems based on nickel complexes of 1,4-diaza-1,3-butadiene (alpha-diimine) ligands in hydrogenation and polymerization reactions. Low Temp. Phys. 2015, 41, 25–28. [Google Scholar] [CrossRef]
- Gao, W.; Xin, L.; Hao, Z.; Li, G.; Su, J.H.; Zhou, L.; Mu, Y. The ligand redox behavior and role in 1,2-bis[(2,6-diisopropylphenyl)imino]-acenaphthene nickel-TMA(MAO) systems for ethylene polymerization. Chem. Commun. 2015, 51, 7004–7007. [Google Scholar] [CrossRef]
- Meinhard, D.; Reuter, P.; Rieger, B. Activation of Polymerization Catalysts: Synthesis and Characterization of Novel Dinuclear Nickel(I) Diimine Complexes. Organometallics 2007, 26, 751–754. [Google Scholar] [CrossRef]
- Leatherman, M.D.; Svejda, S.A.; Johnson, L.K.; Brookhart, M. Mechanistic studies of nickel(II) alkyl agostic cations and alkyl ethylene complexes: Investigations of chain propagation and isomerization in (α-diimine)Ni(II)-catalyzed ethylene polymerization. J. Am. Chem. Soc. 2003, 125, 3068–3081. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; White, P.B.; Hu, C.; Diao, T. Structure and Isotope Effects of the β-H Agostic (α-Diimine)Nickel Cation as a Polymerization Intermediate. Angew. Chem. Int. Ed. 2017, 56, 1535–1538. [Google Scholar] [CrossRef]
- Mohring, V.M.; Fink, G. Novel Polymerization of α-Olefins with the Catalyst System Nickel/Aminobis(imino)phosphorane. Angew. Chem. Int. Ed. 1985, 24, 1001–1003. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. The nature of nickel species formed upon the activation of α-diimine nickel(II) pre-catalyst with alkylaluminum sesquichlorides. J. Organomet. Chem. 2020, 907, 121063. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Luo, G.; Gao, W. Nickel complexes based on BIAN ligands: Transformation and catalysis on ethylene polymerization. Dalton Trans. 2021, 50, 7356–7363. [Google Scholar] [CrossRef] [PubMed]
- Chapleski, R.C., Jr.; Kern, L.; Anderson, W.C., Jr.; Long, B.K.; Roy, S. A Mechanistic Study of Microstructure Modulation in Olefin Polymerizations using a Redox-Active Ni(II) α-Diimine Catalyst. Catal. Sci. Technol. 2020, 10, 2029–2039. [Google Scholar] [CrossRef]
- Zarate, C.; Yang, H.; Bezdek, J.M.; Hesk, D.; Chirik, P.J. Ni(I)-X Complexes Bearing a Bulky α-Diimine Ligand: Synthesis, Structure, and Superior Catalytic Performance in the Hydrogen Isotope Exchange in Pharmaceuticals. J. Am. Chem. Soc. 2019, 141, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Joannou, M.V.; Bezdek, M.J.; Albahily, K.; Korobkov, I.; Chirik, P.J. Synthesis and Reactivity of Reduced α-Diimine Nickel Complexes Relevant to Acrylic Acid Synthesis. Organometallics 2018, 37, 3389–3393. [Google Scholar] [CrossRef]
- Kuang, Y.; Anthony, D.; Katigbak, J.; Marrucci, F.; Humagain, S.; Diao, T. Ni(I)-Catalyzed Reductive Cyclization of 1,6-Dienes: Mechanism-Controlled trans Selectivity. Chem 2017, 3, 268–280. [Google Scholar] [CrossRef]
- Leonard, N.G.; Yruegas, S.; Ho, S.C.; Sattler, A.; Bezdek, M.J.; Chirik, P.J. Synthesis of cationic, dimeric α-diimine nickel hydride complexes and relevance to the polymerization of olefins. Organometallics 2020, 39, 2630–2635. [Google Scholar] [CrossRef]
- Lin, C.Y.; Power, P.P. Complexes of Ni(I): A “rare” oxidation state of growing importance. Chem. Soc. Rev. 2017, 46, 5347–5399. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.C., Jr.; Rhinehart, J.L.; Tennyson, A.G.; Long, B.K. Redox-Active Ligands: An Advanced Tool To Modulate Polyethylene Microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef]
- Kaiser, J.M.; Anderson, W.C., Jr.; Long, B.K. Photochemical regulation of a redox-active olefin polymerization catalyst: Controlling polyethylene microstructure with visible light. Polym. Chem. 2018, 9, 1567–1570. [Google Scholar] [CrossRef]











| Entry | Activator | Activity b | Mnc | Mw/Mn | Branches/1000 C d |
|---|---|---|---|---|---|
| 1 | MMAO | 3.7 | 39 | 1.9 | 40 ± 2 |
| 2 | AlMe3 | 2.7 | 39 | 2.0 | 36 ± 2 |
| 3 e | MMAO | 1.5 | 38 | 2.3 | 39 ± 2 |
| 4 f | AlMe3 | 1.3 | 46 | 2.2 | 36 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Talsi, E.P. α-Diimine Ni-Catalyzed Ethylene Polymerizations: On the Role of Nickel(I) Intermediates. Catalysts 2021, 11, 1386. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111386
Soshnikov IE, Semikolenova NV, Bryliakov KP, Talsi EP. α-Diimine Ni-Catalyzed Ethylene Polymerizations: On the Role of Nickel(I) Intermediates. Catalysts. 2021; 11(11):1386. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111386
Chicago/Turabian StyleSoshnikov, Igor E., Nina V. Semikolenova, Konstantin P. Bryliakov, and Evgenii P. Talsi. 2021. "α-Diimine Ni-Catalyzed Ethylene Polymerizations: On the Role of Nickel(I) Intermediates" Catalysts 11, no. 11: 1386. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111386