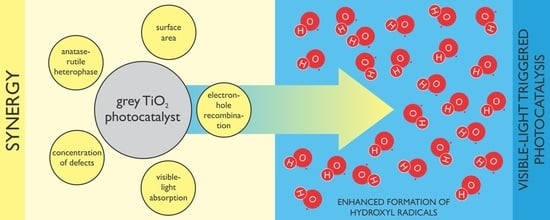
Defective Grey TiO2 with Minuscule Anatase–Rutile Heterophase Junctions for Hydroxyl Radicals Formation in a Visible Light-Triggered Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalyst Characterization
2.1.1. XRD Analysis
2.1.2. Raman Spectroscopy
2.1.3. FTIR Examination
2.1.4. TEM Analysis
2.1.5. UV-Vis DR Analysis
2.1.6. N2 Physisorption Analysis
2.1.7. EPR Examination
2.1.8. Photoluminescence Spectroscopy
2.1.9. XPS Analysis
2.2. Photocatalytic Hydroxyl Radical Formation
3. Materials and Methods
3.1. Synthesis of Photocatalysts
3.1.1. Materials
3.1.2. Synthesis of Pristine and Grey TiO2
3.2. Photocatalyst Characterization
3.3. Photoluminescence Probe Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Fang, X.; Hao, P.; Ren, H.; Zhao, Y.; Huang, F.; Xie, J.; Cui, G.; Tang, B. Controllable fabrication of TiO2 anatase/rutile phase junctions by a designer solvent for promoted photocatalytic performance. Chem. Commun. 2020, 56, 11827–11830. [Google Scholar] [CrossRef]
- Bilecka, I.; Barczuk, P.J.; Augustynski, J. Photoanodic oxidation of small organic molecules at nanostructured TiO2 anatase and rutile film electrodes. Electrochim. Acta 2010, 55, 979–984. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic Activity of Bulk TiO2 Anatase and Rutile Single Crystals Using Infrared Absorption Spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Weng, Y.; Zhou, X.; Wang, X.; Mi, Y.; Chong, R.; Han, H.; Li, C. Achieving overall water splitting using titanium dioxide-based photocatalysts of different phases. Energy Environ. Sci. 2015, 8, 2377–2382. [Google Scholar] [CrossRef]
- Maeda, K. Direct splitting of pure water into hydrogen and oxygen using rutile titania powder as a photocatalyst. Chem. Commun. 2013, 49, 8404–8406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the Relationship between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. 2008, 120, 1790–1793. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xin, Y.; Zhang, Z. Colorful titanium oxides: A new class of photonic materials. Nanoscale 2015, 7, 19894–19898. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Wei, S.; Wu, R.; Xu, X.; Jian, J.; Wang, H.; Sun, Y. One-step synthetic approach for core-shelled black anatase titania with high visible light photocatalytic performance. Chem. Eng. J. 2016, 299, 120–125. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Liu, C.; Zhu, Q.; Zhou, W. Ni2+ and Ti3+ co-doped porous black anatase TiO2 with unprecedented-high visible light-driven photocatalytic degradation performance. RSC Adv. 2015, 5, 107150–107157. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Abdel-Wahab, A. Self-oxygenated anatase–rutile phase junction: Ensuring the availability of sufficient surface charges for photocatalysis. New J. Chem. 2020, 44, 5513–5518. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, Q.; Dai, W.; Ren, Y.; Zhou, Y.; Yang, J.; Xie, S.; Shen, Y.; Wu, J.; Chen, W.; et al. Oxygen-Deficient Blue TiO2 for Ultrastable and Fast Lithium Storage. Adv. Energy Mater. 2020, 10, 1903107. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.-C.; Gong, Y.; Niu, P.; Wang, J.-Q.; Gu, L.; Chen, X.-Q.; Liu, G.; Wang, L.; Cheng, H.-M. An Unusual Strong Visible light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Adv. Mater. 2018, 30, 1704479. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Xu, Y.; Meng, H.; Xu, J.; Wu, J.; Xu, Y.; Zhang, X. Fabrication of polyaniline sensitized grey-TiO2 nanocomposites and enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 184, 248–256. [Google Scholar] [CrossRef]
- Babu, B.; Ullattil, S.G.; Prasannachandran, R.; Kavil, J.; Periyat, P.; Shaijumon, M.M. Ti3+ Induced Brown TiO2 Nanotubes for High Performance Sodium-Ion Hybrid Capacitors. ACS Sustain. Chem. Eng. 2018, 6, 5401–5412. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, M.; Zhang, X.; Cui, N.; Chen, G.; Zou, G.-Y. In-situ nitrogen-doped black TiO2 with enhanced visible light-driven photocatalytic inactivation of Microcystis aeruginosa cells: Synthesization, performance and mechanism. Appl. Catal. B Environ. 2020, 272, 119019. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in Black Titania: A New Material for Advanced Photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ihara, T. Visible light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Band gap engineered, oxygen-rich TiO2 for visible light induced photocatalytic reduction of CO2. Chem. Commun. 2014, 50, 6923–6926. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Goh, B.T.; Mohamed, A.R. Visible light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B Environ. 2015, 179, 160–170. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Photocatalytic reduction of CO2 with H2O over graphene oxide-supported oxygen-rich TiO2 hybrid photocatalyst under visible light irradiation: Process and kinetic studies. Chem. Eng. J. 2017, 308, 248–255. [Google Scholar] [CrossRef]
- Yaemsunthorn, K.; Kobielusz, M.; Macyk, W. TiO2 with Tunable Anatase-to-Rutile Nanoparticles Ratios: How Does the Photoactivity Depend on the Phase Composition and the Nature of Photocatalytic Reaction? ACS Appl. Nano Mater. 2021, 4, 633–643. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.; Ogale, S.B. N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol−Gel Method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Tryba, B.; Toyoda, M.; Morawski, A.W.; Nonaka, R.; Inagaki, M. Photocatalytic activity and OH radical formation on TiO2 in the relation to crystallinity. Appl. Catal. B Environ. 2007, 71, 163–168. [Google Scholar] [CrossRef]
- Liu, N.; Mohajernia, S.; Nguyen, N.T.; Hejazi, S.; Plass, F.; Kahnt, A.; Yokosawa, T.; Osvet, A.; Spiecker, E.; Guldi, D.M.; et al. Long-Living Holes in Grey Anatase TiO2 Enable Noble-Metal-Free and Sacrificial-Agent-Free Water Splitting. ChemSusChem 2020, 13, 4937. [Google Scholar] [CrossRef]
- Fu, F.; Cha, G.; Wu, Z.; Qin, S.; Zhang, Y.; Chen, Y.; Schmuki, P. Photocatalytic Hydrogen Generation from Water-Annealed TiO2 Nanotubes with White and Grey Modification. ChemElectroChem 2021, 8, 240–245. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Nolan, N.T.; Synnott, D.W.; Seery, M.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Gao, P.; Yang, Y.; Yang, P.; Chen, Y.; Wang, Y. N-Doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible light-Driven Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2016, 8, 18126–18131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-K.; Chen, J.-J.; Zhang, X.; Huang, Y.-X.; Li, W.-W.; Yu, H.-Q. Self-induced synthesis of phase-junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Huang, B.; Lu, J.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Hydrogenated titania: Synergy of surface modification and morphology improvement for enhanced photocatalytic activity. Chem. Commun. 2012, 48, 5733–5735. [Google Scholar] [CrossRef] [PubMed]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, L.; Chen, M.; Lu, H.; Zhou, M. Reactions of titanium oxides with water molecules. A matrix isolation FTIR and density functional study. Chem. Phys. Lett. 2001, 343, 178–184. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. Green microwave switching from oxygen rich yellow anatase to oxygen vacancy rich black anatase TiO2 solar photocatalyst using Mn(II) as ‘anatase phase purifier’. Nanoscale 2015, 7, 19184–19192. [Google Scholar] [CrossRef] [PubMed]
- Zavašnik, J.; Šestan, A.; Shvalya, V. Chapter Seven—Microscopic techniques for the characterisation of metal-based nanoparticles. Compr. Anal. Chem. 2021, 93, 241–284. [Google Scholar]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-Doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.-K.; Hu, L.; Zheng, J.; Prabhakaran, D.; Wu, S.; Puchtler, T.J.; Li, M.; Wong, K.-Y.; Taylor, R.A.; et al. Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures. Nat. Commun. 2019, 10, 4421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, R.E.; Knorr, F.J.; McHale, J.L. Comment on “Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study”. J. Phys. Chem. C 2013, 117, 7949–7951. [Google Scholar] [CrossRef]
- Žerjav, G.; Zavašnik, J.; Kovač, J.; Pintar, A. The influence of Schottky barrier height onto visible light triggered photocatalytic activity of TiO2 + Au composites. Appl. Surf. Sci. 2021, 543, 148799. [Google Scholar] [CrossRef]
- Žerjav, G.; Scandura, G.; Garlisi, C.; Palmisano, G.; Pintar, A. Sputtered vs. sol-gel TiO2-doped films: Characterization and assessment of aqueous bisphenol A oxidation under UV and visible light radiation. Catal. Today 2020, 357, 380–391. [Google Scholar] [CrossRef]
- Kawai, T.; Kishimoto, Y.; Kifune, K. Photoluminescence studies of nitrogen-doped TiO2 powders prepared by annealing with urea. Philos. Mag. 2012, 92, 4088–4097. [Google Scholar] [CrossRef]
- Su, T.-Y.; Huang, C.-H.; Shih, Y.-C.; Wang, T.-H.; Medina, H.; Huang, J.-S.; Tsai, H.-S.; Chueh, Y.-L. Tunable defect engineering in TiON thin films by multi-step sputtering processes: From a Schottky diode to resistive switching memory. J. Mater. Chem. C 2017, 5, 6319–6327. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Žerjav, G.; Albreht, A.; Vovk, I.; Pintar, A. Revisiting terephthalic acid and coumarin as probes for photoluminescent determination of hydroxyl radical formation rate in heterogeneous photocatalysis. Appl. Catal. A Gen. 2020, 598, 117566. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase–rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]







| Sample | Mass Fraction of Anatase (%) | Mass Fraction of Rutile (%) | Average Crystallite Size (nm) | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| PT | 100 | 0 | 8.0 | 164 | 0.450 | 10.3 |
| NT-3 | 100 | 0 | 8.1 | 148 | 0.423 | 11.4 |
| NT-4 | 96.6 | 3.4 | 9.4 | 131 | 0.398 | 12.2 |
| NT-5 | 97.2 | 2.8 | 10.3 | 114 | 0.376 | 12.0 |
| NT-O-3 | 100 | 0 | 8.2 | 141 | 0.417 | 11.8 |
| NT-O-4 | 95.8 | 4.2 | 9.3 | 111 | 0.363 | 13.0 |
| NT-O-5 | 96.1 | 3.9 | 11.6 | 85 | 0.294 | 13.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullattil, S.G.; Zavašnik, J.; Maver, K.; Finšgar, M.; Novak Tušar, N.; Pintar, A. Defective Grey TiO2 with Minuscule Anatase–Rutile Heterophase Junctions for Hydroxyl Radicals Formation in a Visible Light-Triggered Photocatalysis. Catalysts 2021, 11, 1500. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121500
Ullattil SG, Zavašnik J, Maver K, Finšgar M, Novak Tušar N, Pintar A. Defective Grey TiO2 with Minuscule Anatase–Rutile Heterophase Junctions for Hydroxyl Radicals Formation in a Visible Light-Triggered Photocatalysis. Catalysts. 2021; 11(12):1500. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121500
Chicago/Turabian StyleUllattil, Sanjay Gopal, Janez Zavašnik, Ksenija Maver, Matjaž Finšgar, Nataša Novak Tušar, and Albin Pintar. 2021. "Defective Grey TiO2 with Minuscule Anatase–Rutile Heterophase Junctions for Hydroxyl Radicals Formation in a Visible Light-Triggered Photocatalysis" Catalysts 11, no. 12: 1500. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121500