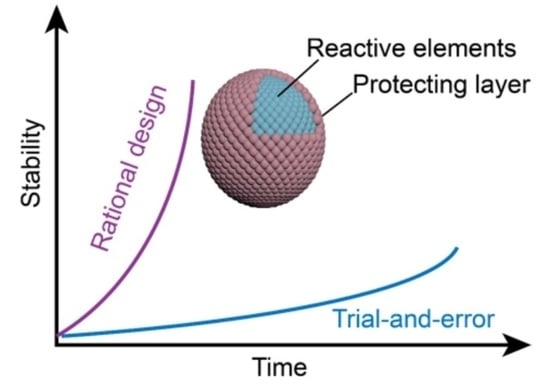
Towards the Rational Design of Stable Electrocatalysts for Green Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICP-MS: | inductively coupled plasma mass spectrometry |
| SEM: | scanning electron microscopy |
| UV-vis spectroscopy: | ultraviolet–visible spectroscopy |
| XPS: | X-ray photoelectron spectroscopy |
| XRD: | X-ray diffraction |
| IL-TEM: | identical location TEM |
| EIS: | electrochemical impedance spectroscopy |
References
- Greeley, J.; Markovic, N.M. The road from animal electricity to green energy: Combining experiment and theory in electrocatalysis. Energy Environ. Sci. 2012, 5, 9246–9256. [Google Scholar] [CrossRef]
- Mergel, J.; Carmo, M.; Fritz, D.; Stolten, D. Transition to Renewable Energy Systems; Stolten, D., Scherer, V., Eds.; Wiley: New York, NY, USA, 2013; pp. 423–450. [Google Scholar]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the stability of oxygen evolution reaction catalysts: The importance of monitoring mass losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef] [Green Version]
- Geiger, S.; Kasian, O.; Mingers, A.M.; Nicley, S.S.; Haenen, K.; Mayrhofer, K.J.J.; Cherevko, S. Catalyst stability benchmarking for the oxygen evolution reaction: The importance of backing electrode material and dissolution in accelerated aging studies. ChemSusChem 2017, 10, 4140–4143. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S. Stability and dissolution of electrocatalysts: Building the bridge between model and “real world” systems. Curr. Opin. Electrochem. 2018, 8, 118–125. [Google Scholar] [CrossRef]
- Kasian, O.; Grote, J.-P.; Geiger, S.; Cherevko, S.; Mayrhofer, K.J.J. The common intermediates of oxygen evolution and dissolution reactions during water electrolysis on iridium. Angew. Chem. Int. Ed. 2018, 57, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Brightman, E.; Dodwell, J.; van Dijk, N.; Hinds, G. In situ characterisation of PEM water electrolysers using a novel reference electrode. Electrochem. Commun. 2015, 52, 1–4. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.T.; Myers, D.; et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef]
- Chen, R.; Hung, S.F.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.B.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered structure causes bulk nife layered double hydroxide unstable in alkaline oxygen evolution reaction. Adv. Mater. 2019, 31, 1903909. [Google Scholar] [CrossRef]
- Cherevko, S.; Reier, T.; Zeradjanin, A.R.; Pawolek, Z.; Strasser, P.; Mayrhofer, K.J.J. Stability of nanostructured iridium oxide electrocatalysts during oxygen evolution reaction in acidic environment. Electrochem. Commun. 2014, 48, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Speck, F.D.; Dettelbach, K.E.; Sherbo, R.S.; Salvatore, D.A.; Huang, A.; Berlinguette, C.P. On the electrolytic stability of iron-nickel oxides. Chem 2017, 2, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Kasian, O.; Geiger, S.; Mayrhofer, K.J.J.; Cherevko, S. Electrochemical on-line ICP-MS in electrocatalysis research. Chem. Rec. 2018, 19, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.B.; Zhang, J.; Chen, J.; Zhang, L.; Xu, Y.; Chen, J.G.; Liu, B. Revealing energetics of surface oxygen redox from kinetic fingerprint in oxygen electrocatalysis. J. Am. Chem. Soc. 2019, 141, 13803–13811. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.B.; Xu, Y.; Huang, X.; Chen, J.; Pei, L.; Zhang, J.; Chen, J.G.; Liu, B. A general method to probe oxygen evolution intermediates at operating conditions. Joule 2019, 3, 1498–1509. [Google Scholar] [CrossRef]
- Kariman, A.; Marshall, A.T. Improving the stability of DSA electrodes by the addition of TiO2 nanoparticles. J. Electrochem. Soc. 2019, 166, E248–E251. [Google Scholar] [CrossRef]
- Tao, H.B.; Fang, L.; Chen, J.; Yang, H.B.; Gao, J.; Miao, J.; Chen, S.; Liu, B. Identification of surface reactivity descriptor for transition metal oxides in oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 9978–9985. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni, Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Sebok, B.; Scott, S.B.; Fiordaliso, E.M.; Sørensen, J.E.; Bodin, A.; Trimarco, D.B.; Damsgaard, C.D.; Vesborg, P.C.K.; Hansen, O.; et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lopes, P.P.; Martins, P.F.B.D.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Wiberg, E.; Nils Wiberg, N.; Holleman, A.F. Inorganic Chemistry; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Li, N.; Araya, S.S.; Kær, S.K. The effect of Fe3+ contamination in feed water on proton exchange membrane electrolyzer performance. Int. J. Hydrog. Energy 2019, 44, 12952–12957. [Google Scholar] [CrossRef]
- Frensch, S.H.; Serre, G.; Fouda-Onana, F.; Jensen, H.C.; Christensen, M.L.; Araya, S.S.; Kær, S.K. Impact of iron and hydrogen peroxide on membrane degradation for polymer electrolyte membrane water electrolysis: Computational and experimental investigation on fluoride emission. J. Power Sources 2019, 420, 54–62. [Google Scholar] [CrossRef]
- Li, N.; Araya, S.S.; Kær, S.K. Long-term contamination effect of iron ions on cell performance degradation of proton exchange membrane water electrolyser. J. Power Sources 2019, 434, 226755. [Google Scholar] [CrossRef]
- Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. Influence of CO2 dissolution into anion exchange membrane on fuel cell performance. Electrochim. Acta 2013, 88, 552–558. [Google Scholar] [CrossRef]
- Matsui, Y.; Saito, M.; Tasaka, A.; Inaba, M. Influence of carbon dioxide on the performance of anion-exchange membrane fuel cells. ECS Trans. 2010, 25, 105–110. [Google Scholar] [CrossRef]
- Ziv, N.; Mondal, A.N.; Weissbach, T.; Holdcroft, S.; Dekel, D.R. Effect of CO2 on the properties of anion exchange membranes for fuel cell applications. J. Membr. Sci. 2019, 586, 140–150. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [Green Version]
- Swierk, J.R.; Klaus, S.; Trotochaud, L.; Bell, A.T.; Tilley, T.D. Electrochemical study of the energetics of the oxygen evolution reaction at nickel iron (oxy)hydroxide catalysts. J. Phys. Chem. C 2015, 119, 19022–19029. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Tang, J.; Zhang, J.; Liu, D.; Zheng, L.; et al. NiFe hydroxide lattice tensile strain: Enhancement of adsorption of oxygenated intermediates for efficient water oxidation catalysis. Angew. Chem. Int. Ed. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Subbaraman, R.; Danilovic, N.; Lopes, P.P.; Tripkovic, D.; Strmcnik, D.; Stamenkovic, V.R.; Markovic, N.M. Origin of anomalous activities for electrocatalysts in alkaline electrolytes. J. Phys. Chem. C 2012, 116, 22231–22237. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Thomas, M.J. Studies of hypervalent iron in aqueous solutions. 1. Radiation-induced reduction of iron(VI) to iron(V) by CO2. J. Am. Chem. Soc. 1987, 109, 7761–7764. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]




| Degradation Mechanism | Characterization Techniques | Signals in Characterization |
|---|---|---|
| Dissolution | ICP-MS, SEM, UV-vis spectroscopy | Morphology, surface area |
| Active site transformation | XPS, XRD | Chemical reactivity of active site |
| Particle growth | TEM, SEM | Morphology, surface area |
| Hydrogen embrittlement | EIS, SEM | Loss in electrical conductivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Bi, S.; Zhang, J.; Tao, H. Towards the Rational Design of Stable Electrocatalysts for Green Hydrogen Production. Catalysts 2022, 12, 204. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020204
Wang X, Bi S, Zhang J, Tao H. Towards the Rational Design of Stable Electrocatalysts for Green Hydrogen Production. Catalysts. 2022; 12(2):204. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020204
Chicago/Turabian StyleWang, Xiangxi, Shengjie Bi, Junming Zhang, and Huabing Tao. 2022. "Towards the Rational Design of Stable Electrocatalysts for Green Hydrogen Production" Catalysts 12, no. 2: 204. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12020204