Catalytic Cracking for Propylene Production over Au Catalyst Supported by External Surface-Modified ZSM-5 Zeolite
Abstract
:1. Introduction
2. Results
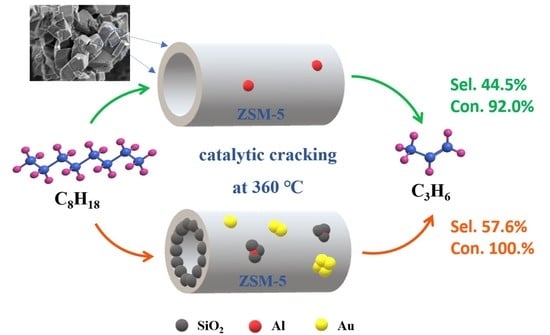
2.1. Catalytic Testing on N-Octane and Light Diesel Oil Cracking
2.2. X-ray Diffraction (XRD) Measurements
2.3. N2 Adsorption−Desorption Isotherms
2.4. Transmission Electron Microscopy (TEM) Observations and EDS Measurements
2.5. NH3 Temperature-Programmed Desorption (TPD) Measurements
2.6. Pyridine Adsorption Fourier Transform Infrared (Py-IR) Measurements
3. Discussion
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Catalyst Characterization
4.3. Catalyst Evaluation for the Catalytic Cracking of N-Octane and Light Diesel
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohei, K.; Hajime, I.; Seitaro, N.; Akira, I. Comparison of steaming stability of Cu-ZSM-5 with those of Ag-ZSM-5, P/H-ZSM-5, and H-ZSM-5 zeolites as naphtha cracking catalysts to produce light olefin at high temperatures. Appl. Catal. A-Gen. 2015, 489, 272–279. [Google Scholar] [CrossRef]
- Alipour, S. Recent advances in naphtha catalytic cracking by nano ZSM-5: A review. Chin. J. Catal. 2016, 37, 671–680. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Wang, G.; Qi, Y.; Xu, L.; Xie, P.; He, Y. Production of light olefins and aromatic hydrocarbons through catalytic cracking of naphtha at lowered temperature. Stud. Surf. Sci. Catal. 2005, 158, 1223–1230. [Google Scholar] [CrossRef]
- Lin, L.F.; Qiu, C.F.; Zhuo, Z.X.; Zhang, D.W.; Zhao, S.F.; Wu, H.H.; Liu, Y.M.; He, M.Y. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A-Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Konno, H.; Tago, T.; Nakasaka, Y.; Ohnaka, R.; Nishimura, J.; Masuda, T. Effectiveness of nano-scale ZSM-5 zeolite and its deactivation mechanism on catalytic cracking of representative hydrocarbons of naphtha. Microporous Mesoporous Mater. 2013, 175, 25–33. [Google Scholar] [CrossRef]
- Panya, W.; Prasert, R.; Naoki, M.; Osamu, S.; Aritomo, Y. Effect of carbon number on the production of propylene and ethylene by catalytic cracking of straight-chain alkanes over phosphorus-modified ZSM-5. Fuel Process. Technol. 2020, 202, 106367. [Google Scholar] [CrossRef]
- Isabelle, B.; Alexander, S. Determination of the exact microporous volume and BET surface area in hierarchical ZSM–5. J. Phys. Chem. C. 2019, 123, 4235–4242. [Google Scholar] [CrossRef]
- Wang, C.F.; Zhang, L.; Huang, X.; Zhu, Y.F.; Li, G.; Gu, Q.F.; Chen, J.Y.; Ma, L.G.; Li, X.J.; He, Q.H.; et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.L.; Shaikh, S.; Vittenet, J.R.; Wang, J.J.; Liu, Z.H.; Bhatte, K.D.; Ali, O.; Xu, W.; Osorio, I.; Saih, Y.; et al. Fine tuning the diffusion length in hierarchical ZSM-5 to maximize the yield of propylene in catalytic cracking of hydrocarbons. ACS Sustain. Chem. Eng. 2018, 6, 15832–15840. [Google Scholar] [CrossRef]
- Inagaki, S.; Sato, K.; Hayashi, S.; Tatami, J.; Kubota, Y.; Wakihara, T. A mechanochemical approach for selective deactivation of external surface acidity of ZSM-5 zeolite catalyst. ACS Appl. Mater. Interfaces 2015, 7, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Knöll, J.; Singh, U.; Nicolich, J.; Gonzalez, R.; Ziebarth, M.; Fougret, C.; Brandt, S. Unit cell volume as a measure of dealumination of ZSM 5 in fluid catalytic cracking catalyst. Ind. Eng. Chem. Res. 2014, 53, 16270–16274. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.L.; Ma, J.H.; Li, R.F.; Jiao, H.J. Determining the structures, acidity and adsorption properties of Al substituted HZSM-5. Phys. Chem. Chem. Phys. 2019, 21, 18758–18768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, J.S.; Xu, C.M.; Shen, B.J. Alkali-treatment of ZSM-5 zeolites with different SiO2/Al2O3 ratios and light olefin production by heavy oil cracking. Fuel Process. Technol. 2011, 92, 414–420. [Google Scholar] [CrossRef]
- Wan, J.L.; Wei, Y.X.; Liu, Z.M.; Li, B.; Qi, Y.; Li, M.Z.; Xie, P.; Meng, S.H.; He, Y.L.; Chang, F.X. A ZSM-5-based catalyst for efficient production of light olefins and aromatics from fluidized-bed naphtha catalytic cracking. Catal. Lett. 2008, 124, 150–156. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.; Seo, G. Catalytic cracking of n-octane over alkali-treated MFI zeolites. Appl. Catal. A-Gen. 2005, 288, 149–157. [Google Scholar] [CrossRef]
- Su, L.L.; Liu, L.; Zhuang, J.Q.; Wang, H.X.; Li, Y.G.; Shen, W.J.; Xu, Y.D.; Bao, X.H. Creating mesopores in ZSM-5 zeolite by alkali treatment: A new way to enhance the catalytic performance of methane dehydroaromatization on Mo/HZSM-5 catalysts. Catal. Lett. 2003, 91, 155–167. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.L.; Hu, X.L.; Zhang, Y.X.; Wu, J.J.; Yan, Z.F. Phosphorus-modified b-axis oriented hierarchical ZSM-5 zeolites for enhancing catalytic performance in a methanol to propylene reaction. Appl. Catal. A-Gen. 2020, 594, 117464. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Sun, L.B.; Su, H.J.; Zou, X.H.; Qi, C.X. The performance of catalytic conversion of ZSM-5 comodified with gold and lanthanum for increasing propylene production. Ind. Eng. Chem. Res. 2019, 58, 14695–14704. [Google Scholar] [CrossRef]
- Zhang, C.D.; Kwak, G.; Lee, Y.J.; Jun, K.W.; Gao, R.X.; Park, H.G.; Kim, S.; Min, J.E.; Chang Kang, S.; Guan, G.F. Light hydrocarbons to BTEX aromatics over Zn-modified hierarchical ZSM-5 combined with enhanced catalytic activity and stability. Microporous Mesoporous Mater. 2019, 284, 316–326. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Gao, X.Z.; Zhang, X.; Xu, G.T.; Li, G.C.; Zheng, A.M. A theoretical study the effective phosphorous species over modified P-ZSM-5 zeolite. Phys. Chem. Chem. Phys. 2018, 20, 11702–11712. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Qiu, Y.; Tian, Y.J.; Diao, Z.H.; Zhang, X.W.; Liu, G.Z. Reaction pathways of n-pentane cracking on the fresh and regenerated Sr, Zr and La-loaded ZSM-5 zeolites. Chem. Eng. J. 2018, 349, 297–308. [Google Scholar] [CrossRef]
- Dai, W.L.; Yang, L.; Wang, C.M.; Wang, X.; Wu, G.J.; Guan, N.J.; Obenaus, U.; Hunger, M.; Li, L.D. Effect of n-butanol cofeeding on the methanol to aromatics conversion over Ga-modified nano H-ZSM-5 and its mechanistic interpretation. ACS Catal. 2018, 8, 1352–1362. [Google Scholar] [CrossRef]
- Long, L.; Lan, Z.Z.; Han, Z.X.; Qiu, Y.F.; Zhou, W.X. Co3O4 nanosheet wrapped commercial HZSM-5 for promoting catalytic cracking of n-decane and anticoking activities. ACS Appl. Energy Mater. 2018, 1, 4130–4139. [Google Scholar] [CrossRef]
- Li, F.W.; Ding, S.L.; Wang, Z.H.; Li, Z.X.; Li, L.; Gao, C.; Zhong, Z.; Lin, H.F.; Chen, C.J. Production of light olefins from catalytic cracking biooil model compounds over La2O3-modified ZSM-5 zeolite. Energy Fuel 2018, 32, 5910–5922. [Google Scholar] [CrossRef]
- Qi, C.X.; Wang, Y.X.; Ding, X.T.; Su, H.J. Catalytic cracking of light diesel over Au/ZSM-5 catalyst for increasing propylene production. Chin. J. Catal. 2016, 37, 1747–1754. [Google Scholar] [CrossRef]
- Ai Sha, N.L.H.; Liu, J.X.; He, N.; Guo, H.C. Catalytic conversion of n-butane over Au-Zn-modified nano-sized HZSM-5. Chin. J. Catal. 2013, 34, 1262–1266. [Google Scholar] [CrossRef]
- Sami, M.; Brahim, M.; Pieter, C.; Evgeny, A.; Emiel, J. Structure and reactivity of Zn-modified ZSM-5 zeolites: The importance of clustered cationic Zn complexes. ACS Catal. 2012, 2, 71–83. [Google Scholar] [CrossRef]
- Corma, A.; Mengual, J.; Miguelc, P. Stabilization of ZSM-5 zeolite catalysts for steam catalytic cracking of naphtha for production of propene and ethene. Appl. Catal. A-Gen. 2012, 421−422, 121–134. [Google Scholar] [CrossRef]
- Siddiqui, M.; Aitani, A.; Saeed, M.; Al-Yassir, N.; Al-Khattaf, S. Enhancing propylene production from catalytic cracking of Arabian Light VGO over novel zeolites as FCC catalyst additives. Fuel 2011, 90, 459–466. [Google Scholar] [CrossRef]
- Xue, N.H.; Liu, N.; Nie, L.; Yu, Y.; Gu, M.; Peng, L.M.; Guo, X.F.; Ding, W.P. 1-Butene cracking to propene over P/HZSM-5: Effect of lanthanum. J. Mol. Catal. A-Chem. 2010, 327, 12–19. [Google Scholar] [CrossRef]
- Wang, X.N.; Zhao, Z.; Xu, C.M.; Duan, A.J.; Zhang, L.; Jiang, G.Y. Effects of light rare earth on acidity and catalytic performance of HZSM-5 zeolite for catalytic clacking of butane to light olefins. J. Rare Earths 2007, 25, 321–328. [Google Scholar] [CrossRef]
- Niwa, M.; Murakami, Y. CVD zeolites with controlled pore-opening size. J. Phys. Chem. Solids. 1989, 50, 487–496. [Google Scholar] [CrossRef]
- Niwa, M.; Kota, M.; Hattori, T.; Murakami, Y. Fine control of the pore-opening size of zeolite ZSM-5 by chemical vapor deposition of silicon methoxide. J. Phys. Chem. 1986, 90, 6233–6237. [Google Scholar] [CrossRef]
- Niwa, M.; Kawashima, Y.; Murakam, Y. A shape-selective platinum-loaded mordenite catalyst for the hydrocracking of paraffins by the chemical vapour deposition of silicon alkoxide. J. Chem. Soc. Faraday. Trans. 1 1985, 81, 2757–2761. [Google Scholar] [CrossRef]
- Niwa, M.; Kato, S.; Hattori, T.; Murakam, Y. Fine control of the pore-opening size of the zeolite mordenite by chemical vapour deposition of silicon alkoxide. J. Chem. Soc. Faraday. Trans. 1 1984, 80, 3135–3145. [Google Scholar] [CrossRef]
- Niwa, M.; Itoh, H.; Kato, S.; Hattori, T.; Murakami, Y. Modification of H-mordenite by a vapour-phase deposition method. J. Chem. Soc. Chem. Commun. 1982, 245, 819–820. [Google Scholar] [CrossRef]
- Yue, Y.H.; Tang, Y.; Gao, Z. Zeolite pore size engineering by chemical liquid deposition. Stud. Surf. Sci. Catal. 1997, 105, 2059–2065. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Wang, Z.P.; Gilbert, C.J.; Fan, W.; Huber, G.W. Production of p-xylene from biomass by catalytic fast pyrolysis using ZSM-5 catalysts with reduced pore openings. Angew. Chem. Int. Ed. 2012, 51, 11097–11100. [Google Scholar] [CrossRef]
- Hayashi, T.; Tanaka, K.; Haruta, M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen. J. Catal. 1998, 178, 566–575. [Google Scholar] [CrossRef]
- Qi, C.X.; Zheng, Y.H.; Lin, H.; Su, H.J.; Sun, X.; Sun, L.B. CO oxidation over gold catalysts supported on CuO/Cu2O both in O2-richand H2-rich streams: Necessity of copper oxide. Appl. Catal. B-Environ. 2019, 253, 160–169. [Google Scholar] [CrossRef]
- Qi, C.X.; Zhu, S.D.; Su, H.J.; Lin, H.; Guan, R.G. Stability improvement of Au/Fe-La-Al2O3 catalyst by incorporated with a FexOy layer in CO oxidation process. Appl. Catal. B-Environ. 2013, 138−139, 104–112. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tian, Y.J.; Zhang, B.F.; Liang, H.R.; Hou, X.; Wang, L.; Zhang, X.W.; Liu, G.Z. Synthesis and performance of pillared HZSM-5 nanosheet zeolites for n-decane catalytic cracking to produce light olefins. Appl. Catal. A-Gen. 2019, 572, 24–33. [Google Scholar] [CrossRef]
- Wang, F.; Chu, X.Z.; Zhao, P.S.; Zhu, F.X.; Li, Q.Q.; Wu, F.Y.; Xiao, G.M. Shape selectivity conversion of biomass derived glycerol to aromatics over hierarchical HZSM-5 zeolites prepared by successive steaming and alkaline leaching: Impact of acid properties and pore constraint. Fuel 2020, 262, 116538. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Long, H.A.; Sun, L.B.; Murayama, T.; Qi, C.X. Insights into au nanoparticle size and chemical state of Au/ZSM-5 catalyst for catalytic cracking of n-octane to increase propylene production. J. Phys. Chem. C 2021, 125, 16013–16023. [Google Scholar] [CrossRef]
- Corma, A.; Orchilles, A.V. Current views on the mechanism of catalytic cracking. Microporous Mesoporous Mater. 2000, 35−36, 21–30. [Google Scholar] [CrossRef]
- Wang, C.Y.; Garbarino, G.; Allard, L.F.; Wilson, F.; Busca, G.; Flytzani-Stephanopoulos, M. Low-temperature dehydrogenation of ethanol on atomically dispersed gold supported on ZnZrOx. ACS Catal. 2016, 6, 210–218. [Google Scholar] [CrossRef]
- Zheng, J.W.; Qu, J.; Lin, H.Q.; Zhang, Q.; Yuan, X.; Yang, Y.H.; Yuan, Y.Z. Surface composition control of the binary Au−Ag catalyst for enhanced oxidant-free dehydrogenation. ACS Catal. 2016, 6, 6662–6669. [Google Scholar] [CrossRef]







| Catalyst | Temperature (°C) | Product Distribution (%) | Conversion (%) | MAT (%) | Propylene Selectivity (%) | ||
|---|---|---|---|---|---|---|---|
| Dry Gas+LPG a | Gasoline | Diesel Oil | |||||
| Z-before | 360 | 47.8 | 6.6 | 45.6 | 53.5 | 54.5 | 42.6 |
| 410 | 45.8 | 5.8 | 48.4 | 50.6 | 51.6 | 44.5 | |
| 460 | 46.8 | 5.7 | 47.5 | 51.5 | 52.5 | 47.2 | |
| 0.2Au/Z-1T-before | 360 | 44.7 | 10.4 | 44.9 | 54.2 | 55.1 | 42.9 |
| 410 | 46.8 | 9.1 | 44.1 | 55.0 | 55.9 | 46.6 | |
| 460 | 47.9 | 7.7 | 44.4 | 54.7 | 55.6 | 48.7 | |
| Z-after | 360 | 34.0 | 1.7 | 64.3 | 34.4 | 35.7 | 46.4 |
| 410 | 34.0 | 1.2 | 64.8 | 33.9 | 35.3 | 57.2 | |
| 460 | 36.2 | 1.3 | 62.5 | 36.1 | 37.5 | 56.8 | |
| 0.2Au/Z-1T-after | 360 | 38.3 | 3.7 | 58.0 | 40.8 | 42.0 | 46.6 |
| 410 | 39.4 | 1.8 | 58. 8 | 39.9 | 41.1 | 58.2 | |
| 460 | 35.1 | 2.3 | 62.6 | 36.1 | 37.4 | 59.2 | |
| Sample | Total Acidity /mmol·g−1 | Strong Acidity /mmol·g−1 | Medium Acidity /mmol·g−1 | Weak Acidity /mmol·g−1 |
|---|---|---|---|---|
| Z | 0.192 | 0.051 | 0.074 | 0.067 |
| Z-5-1T | 0.180 | 0.029 | 0.068 | 0.083 |
| 0.1Au/Z-0.5T | 0.179 | 0.035 | 0.069 | 0.075 |
| 0.1Au/Z-1T | 0.190 | 0.032 | 0.078 | 0.080 |
| 0.1Au/Z-2T | 0.207 | 0.026 | 0.084 | 0.097 |
| 0.1Au/Z-3T | 0.155 | 0.019 | 0.053 | 0.083 |
| 0.1Au/Z-4T | 0.145 | 0.015 | 0.053 | 0.077 |
| 0.2Au/Z-1T | 0.165 | 0.023 | 0.058 | 0.084 |
| 0.3Au/Z-1T | 0.237 | 0.042 | 0.081 | 0.114 |
| 0.4Au/Z-1T | 0.218 | 0.039 | 0.071 | 0.108 |
| 0.5Au/Z-1T | 0.235 | 0.040 | 0.078 | 0.117 |
| Sample | CB/umol·g−1 | CL/umol·g−1 | CB/CL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 °C | 300 °C | 450 °C | 150 °C | 300 °C | 450 °C | 150 °C | 300 °C | 450 °C | |
| Z | 303.4 | 191.1 | 111.3 | 251.6 | 121.7 | 30.4 | 1.21 | 1.57 | 3.72 |
| Z-1T | 176.1 | 129.4 | 67.2 | 260.6 | 112.4 | 34.3 | 0.68 | 1.15 | 1.96 |
| 0.1Au/Z-1T | 210.6 | 81.8 | 48.3 | 205.9 | 79.4 | 28.2 | 1.02 | 1.02 | 1.71 |
| 0.2Au/Z-1T | 242.9 | 99.2 | 23.8 | 206.3 | 88.6 | 30.1 | 1.18 | 1.12 | 0.79 |
| Sample | Si2p Atomic (%) | Al2p Atomic (%) | O1s Atomic (%) |
|---|---|---|---|
| Z | 25.95 | 2.75 | 55.92 |
| Z-1T | 26.60 | 2.69 | 57.47 |
| Z-4T | 26.75 | 2.43 | 57.29 |
| 0.2Au/Z-1T | 27.14 | 2.30 | 57.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Su, H.; Sun, X.; Sun, L.; Zhao, L.; Qi, C. Catalytic Cracking for Propylene Production over Au Catalyst Supported by External Surface-Modified ZSM-5 Zeolite. Catalysts 2022, 12, 418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12040418
Wu L, Su H, Sun X, Sun L, Zhao L, Qi C. Catalytic Cracking for Propylene Production over Au Catalyst Supported by External Surface-Modified ZSM-5 Zeolite. Catalysts. 2022; 12(4):418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12040418
Chicago/Turabian StyleWu, Lei, Huijuan Su, Xun Sun, Libo Sun, Lijun Zhao, and Caixia Qi. 2022. "Catalytic Cracking for Propylene Production over Au Catalyst Supported by External Surface-Modified ZSM-5 Zeolite" Catalysts 12, no. 4: 418. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12040418