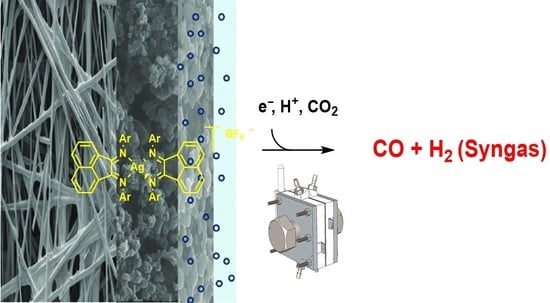
Tuning the Electronic Properties of Homoleptic Silver(I) bis-BIAN Complexes towards Efficient Electrocatalytic CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Electrochemical Characterization
2.3. Homogeneous Electrocatalysis
2.4. Heterogeneous Electrocatalysis
3. Materials and Methods
3.1. Synthesis and Characterization
3.1.1. General Procedure for the Preparation of BIAN Ligands
1-Pyrene-BIAN (L3)
4-Diethylamino-BIAN (L6)
3,4,5-Trimethoxy-BIAN (L5)
3.1.2. General Procedure for the Preparation of [Ag(I)(BIAN)2]BF4 Complexes Ag1–Ag6
3.2. Homogeneous Electrochemistry
3.3. Heterogeneous Electrochemistry
3.3.1. Fabrication of Gas Diffusion Electrodes
3.3.2. Electrochemical Measurements
3.3.3. Product Analysis
Calculation of the Faradaic Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, H.; Li, X.; Meng, J.; Zheng, H.; Zhang, W.; Cao, R. Structure Effects of Metal Corroles on Energy-Related Small Molecule Activation Reactions. ACS Catal. 2019, 9, 4320–4344. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Lu, J.; Yan, Q.; Yuan, W.; Li, C.M. Three-Dimensional Ni Foam-Supported CoO Nanoparticles/N-Doped Carbon Multilayer Nanocomposite Electrode for Oxygen Evolution. ACS Appl. Nano Mater. 2020, 3, 11416–11425. [Google Scholar] [CrossRef]
- Wang, X.; Fei, Y.; Wang, W.; Yuan, W.; Li, C.M. Polymer-Mediated Self-Assembly of Amorphous Metal–Organic Complexes toward Fabrication of Three-Dimensional Graphene Supported CoP Nanoparticle-Embedded N-Doped Carbon as a Superior Hydrogen Evolution Catalyst. ACS Appl. Energy Mater. 2019, 2, 8851–8861. [Google Scholar] [CrossRef]
- Yuan, W.; Li, C.; Zhao, M.; Zhang, J.; Li, C.M.; Jiang, S.P. In situ self-assembled 3-D interconnected pristine graphene supported NiO nanosheets as superior catalysts for oxygen evolution. Electrochim. Acta 2020, 342, 136118. [Google Scholar] [CrossRef]
- Pegis, M.L.; Wise, C.F.; Martin, D.J.; Mayer, J.M. Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. [Google Scholar] [CrossRef] [PubMed]
- Miner, E.M.; Fukushima, T.; Sheberla, D.; Sun, L.; Surendranath, Y.; Dincă, M. Electrochemical oxygen reduction catalysed by Ni3(hexaiminotriphenylene)2. Nat. Commun. 2016, 7, 10942. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Shin, H.; Hooch Antink, W.; Sung, Y.-E.; Hyeon, T. Recent Advances in Electrochemical Oxygen Reduction to H2O2: Catalyst and Cell Design. ACS Energy Lett. 2020, 5, 1881–1892. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R.; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, Photons, Protons and Earth-Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction. ACS Catal. 2017, 7, 70–88. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Dedić, D.; Dorniak, A.; Rinner, U.; Schöfberger, W. Recent Progress in (Photo-)-Electrochemical Conversion of CO2 With Metal Porphyrinoid-Systems. Front. Chem. 2021, 9, 685619. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over Catalytic Surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef] [Green Version]
- Kinzel, N.W.; Demirbas, D.; Bill, E.; Weyhermüller, T.; Werlé, C.; Kaeffer, N.; Leitner, W. Systematic Variation of 3d Metal Centers in a Redox-Innocent Ligand Environment: Structures, Electrochemical Properties, and Carbon Dioxide Activation. Inorg. Chem. 2021, 60, 19062–19078. [Google Scholar] [CrossRef]
- Shen, J.; Kortlever, R.; Kas, R.; Birdja, Y.Y.; Diaz-Morales, O.; Kwon, Y.; Ledezma-Yanez, I.; Schouten, K.J.P.; Mul, G.; Koper, M.T.M. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 2015, 6, 8177. [Google Scholar] [CrossRef]
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef]
- Kinzel, N.W.; Werlé, C.; Leitner, W. Transition Metal Complexes as Catalysts for the Electroconversion of CO2: An Organometallic Perspective. Angew. Chem. Int. Ed. 2021, 60, 11628–11686. [Google Scholar] [CrossRef]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E.; et al. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Portenkirchner, E.; Kianfar, E.; Sariciftci, N.S.; Knör, G. Two-electron carbon dioxide reduction catalyzed by rhenium(I) bis(imino)acenaphthene carbonyl complexes. ChemSusChem 2014, 7, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Todorova, T.K.; Fontecave, M.; Mougel, V. Electroreduction of CO2 to Formate with Low Overpotential using Cobalt Pyridine Thiolate Complexes. Angew. Chem. Int. Ed. 2020, 59, 15726–15733. [Google Scholar] [CrossRef] [PubMed]
- Kopljar, D.; Inan, A.; Vindayer, P.; Wagner, N.; Klemm, E. Electrochemical reduction of CO2 to formate at high current density using gas diffusion electrodes. J. Appl. Electrochem. 2014, 44, 1107–1116. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487. [Google Scholar] [CrossRef]
- Gonglach, S.; Paul, S.; Haas, M.; Pillwein, F.; Sreejith, S.S.; Barman, S.; De, R.; Müllegger, S.; Gerschel, P.; Apfel, U.-P.; et al. Molecular cobalt corrole complex for the heterogeneous electrocatalytic reduction of carbon dioxide. Nat. Commun. 2019, 10, 3864. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Barile, C.J. Electrochemical CO2 reduction to methane with remarkably high Faradaic efficiency in the presence of a proton permeable membrane. Energy Environ. Sci. 2020, 13, 3567–3578. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Wang, J.; Ling, C.; Niu, W.; Huang, Z.; Liu, G.; Chen, B.; Lai, Z.; Liu, X.; et al. Ethylene Selectivity in Electrocatalytic CO2 Reduction on Cu Nanomaterials: A Crystal Phase-Dependent Study. J. Am. Chem. Soc. 2020, 142, 12760–12766. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef]
- Ogura, K. Electrochemical reduction of carbon dioxide to ethylene: Mechanistic approach. J. CO2 Util. 2013, 1, 43–49. [Google Scholar] [CrossRef]
- De, R.; Gonglach, S.; Paul, S.; Haas, M.; Sreejith, S.S.; Gerschel, P.; Apfel, U.-P.; Vuong, T.H.; Rabeah, J.; Roy, S.; et al. Electrocatalytic Reduction of CO2 to Acetic Acid by a Molecular Manganese Corrole Complex. Angew. Chem. Int. Ed. 2020, 59, 10527–10534. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Abramov, P.A.; Dmitriev, A.A.; Kholin, K.V.; Gritsan, N.P.; Kadirov, M.K.; Gushchin, A.L.; Sokolov, M.N. Mechanistic study of the [(dpp-bian)Re(CO)3Br] electrochemical reduction using in situ EPR spectroscopy and computational chemistry. Electrochim. Acta 2018, 270, 526–534. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Fang, X.; Li, H.; Zhang, Z.; Hor, T.S.A.; Weng, Z. Aryl-BIAN-ligated silver(I) trifluoromethoxide complex. Dalton Trans. 2015, 44, 19682–19686. [Google Scholar] [CrossRef]
- Papanikolaou, P.A.; Gdaniec, M.; Wicher, B.; Akrivos, P.D.; Tkachenko, N.V. Bis(aryl)acenaphthenequinonediimine Substituent Effect on the Properties and Coordination Environment of Ligands and Their Bis-Chelate AgI Complexes. Eur. J. Inorg. Chem. 2013, 2013, 5196–5205. [Google Scholar] [CrossRef]
- Rosa, V.; Santos, C.I.M.; Welter, R.; Aullón, G.; Lodeiro, C.; Avilés, T. Comparison of the structure and stability of new α-diimine complexes of copper(I) and silver(I): Density functional theory versus experimental. Inorg. Chem. 2010, 49, 8699–8708. [Google Scholar] [CrossRef]
- Gasperini, M.; Ragaini, F.; Cenini, S. Synthesis of Ar-BIAN Ligands (Ar-BIAN = Bis(aryl)acenaphthenequinonediimine) Having Strong Electron-Withdrawing Substituents on the Aryl Rings and Their Relative Coordination Strength toward Palladium(0) and -(II) Complexes. Organometallics 2002, 21, 2950–2957. [Google Scholar] [CrossRef]
- Scarel, A.; Axet, M.R.; Amoroso, F.; Ragaini, F.; Elsevier, C.J.; Holuigue, A.; Carfagna, C.; Mosca, L.; Milani, B. Subtle Balance of Steric and Electronic Effects for the Synthesis of Atactic Polyketones Catalyzed by Pd Complexes with Meta-Substituted Aryl-BIAN Ligands. Organometallics 2008, 27, 1486–1494. [Google Scholar] [CrossRef]
- Rosa, V.; Avilés, T.; Aullon, G.; Covelo, B.; Lodeiro, C. A new bis(1-naphthylimino)acenaphthene compound and its Pd(II) and Zn(II) complexes: Synthesis, characterization, solid-state structures and density functional theory studies on the syn and anti isomers. Inorg. Chem. 2008, 47, 7734–7744. [Google Scholar] [CrossRef] [PubMed]
- Löw, I.; Bubrin, M.; Paretzki, A.; Fiedler, J.; Záliš, S.; Kaim, W. The BIAN ligand 1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene: An electron sponge or a “normal” α-diimine ligand? Inorg. Chim. Acta 2017, 455, 540–548. [Google Scholar] [CrossRef]
- Hasan, K.; Zysman-Colman, E. Synthesis, UV-Vis and CV properties of a structurally related series of bis(Arylimino)acenaphthenes (Ar-BIANs). J. Phys. Org. Chem. 2013, 26, 274–279. [Google Scholar] [CrossRef]
- El-Ayaan, U.; Murata, F.; El-Derby, S.; Fukuda, Y. Synthesis, structural and solvent influence studies on solvatochromic mixed-ligand copper(II) complexes with the rigid nitrogen ligand: Bis[N-(2,4,6-trimethylphenyl)imino]acenaphthene. J. Mol. Struct. 2004, 692, 209–216. [Google Scholar] [CrossRef]
- Kern, T.; Monkowius, U.; Zabel, M.; Knör, G. Synthesis, crystal structure and charge transfer spectra of dinuclear copper(I) complexes bearing 1,2-bis(arylimino)acenaphthene acceptor ligands. Inorg. Chim. Acta 2011, 374, 632–636. [Google Scholar] [CrossRef]
- El-Ayaan, U.; Paulovicova, A.; Fukuda, Y. Structural studies of mixed-ligands copper(II) and copper(I) complexes with the rigid nitrogen ligand: Bis[N-(2,6-diisopropylphenyl)imino]acenaphthene. J. Mol. Struct. 2003, 645, 205–212. [Google Scholar] [CrossRef]
- Coventry, D.N.; Batsanov, A.S.; Goeta, A.E.; Howard, J.A.; Marder, T.B. Synthesis and molecular structures of α-diimines and their zinc and palladium dichloride complexes. Polyhedron 2004, 23, 2789–2795. [Google Scholar] [CrossRef]
- Aranzaes, J.R.; Daniel, M.-C.; Astruc, D. Metallocenes as references for the determination of redox potentials by cyclic voltammetry—Permethylated iron and cobalt sandwich complexes, inhibition by polyamine dendrimers, and the role of hydroxy-containing ferrocenes. Can. J. Chem. 2006, 84, 288–299. [Google Scholar] [CrossRef]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Tsierkezos, N.G. Cyclic Voltammetric Studies of Ferrocene in Nonaqueous Solvents in the Temperature Range from 248.15 to 298.15 K. J. Solut. Chem. 2007, 36, 289–302. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Chudakova, V.A.; Fukin, G.K. Four-step reduction of dpp-bian with sodium metal: Crystal structures of the sodium salts of the mono-, di-, tri- and tetraanions of dpp-bian. Angew. Chem. Int. Ed. 2003, 42, 3294–3298. [Google Scholar] [CrossRef]
- Hill, N.J.; Vargas-Baca, I.; Cowley, A.H. Recent developments in the coordination chemistry of bis(imino)acenaphthene (BIAN) ligands with s- and p-block elements. Dalton Trans. 2009, 240–253. [Google Scholar] [CrossRef]
- Li, L.; Lopes, P.S.; Rosa, V.; Figueira, C.A.; Lemos, M.A.N.D.A.; Duarte, M.T.; Avilés, T.; Gomes, P.T. Synthesis and structural characterisation of (aryl-BIAN)copper(I) complexes and their application as catalysts for the cycloaddition of azides and alkynes. Dalton Trans. 2012, 41, 5144–5154. [Google Scholar] [CrossRef]
- Papanikolaou, P.; Akrivos, P.D.; Czapik, A.; Wicher, B.; Gdaniec, M.; Tkachenko, N. Homoleptic Bis(aryl)acenaphthenequinonediimine–CuI Complexes—Synthesis and Characterization of a Family of Compounds with Improved Light-Gathering Characteristics. Eur. J. Inorg. Chem. 2013, 2013, 2418–2431. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A.; et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. [Google Scholar] [CrossRef]
- Gohar, G.A.; Habeeb, M.M. Proton transfer equilibria, temperature and substituent effects on hydrogen bonded complexes between chloranilic acid and anilines. Spectroscopy 2000, 14, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Jitaru, M. Electrochemical carbon dioxide reduction—Fundamental and applied topics. J. Chem. Technol. Metall. 2007, 42, 333–344. [Google Scholar]
- Gennaro, A.; Isee, A.A.; Vianello, E. Solubility and electrochemical determination of CO2 in some dipolar aprotic solvents. J. Electroanal. Chem. 1990, 289, 203–215. [Google Scholar] [CrossRef]
- Kaiser, U.; Heitz, E. Zum Mechanismus der elektrochemischen Dimerisierung von CO2 zu Oxalsäure. Ber. Bunsenges. Phys. Chem. 1973, 77, 818–823. [Google Scholar] [CrossRef]
- König, M.; Vaes, J.; Klemm, E.; Pant, D. Solvents and Supporting Electrolytes in the Electrocatalytic Reduction of CO2. iScience 2019, 19, 135–160. [Google Scholar] [CrossRef]
- Sullivan, B.P. Electrochemical and Electrocatalytic Reactions of Carbon Dioxide; Elsevier Science: Burlington, ON, Canada, 1992; ISBN 9780444596611. [Google Scholar]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Kochi, J.K. Continuum of outer- and inner-sphere mechanisms for organic electron transfer. Steric modulation of the precursor complex in paramagnetic (ion-radical) self-exchanges. J. Am. Chem. Soc. 2007, 129, 3683–3697. [Google Scholar] [CrossRef] [PubMed]
- Savéant, J.-M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 2008, 108, 2348–2378. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.V.R.; Fianchini, M. A classical silver carbonyl complex {MeB[3-(Mes)pz]3}Ag(CO) and the related silver ethylene adduct {MeB[3-(Mes)pz]3}Ag(C2H4). Angew. Chem. Int. Ed. 2007, 46, 2188–2191. [Google Scholar] [CrossRef]
- Wan, Q.; He, Q.; Zhang, Y.; Zhang, L.; Li, J.; Hou, J.; Zhuang, X.; Ke, C.; Zhang, J. Boosting the faradaic efficiency for carbon dioxide to monoxide on a phthalocyanine cobalt based gas diffusion electrode to higher than 99% via microstructure regulation of catalyst layer. Electrochim. Acta 2021, 392, 139023. [Google Scholar] [CrossRef]
- Jaster, T.; Gawel, A.; Siegmund, D.; Holzmann, J.; Lohmann, H.; Klemm, E.; Apfel, U.-P. Electrochemical CO2 reduction toward multicarbon alcohols—The microscopic world of catalysts & process conditions. iScience 2022, 25, 104010. [Google Scholar] [CrossRef] [PubMed]
- Pellumbi, K.; Smialkowski, M.; Siegmund, D.; Apfel, U.-P. Enhancing the CO2 Electroreduction of Fe/Ni-Pentlandite Catalysts by S/Se Exchange. Chemistry 2020, 26, 9938–9944. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, D.; Pellumbi, K.; Puring, K.j.; Siegmund, D.; Polet, W.S.K.; Checinski, M.P.; Apfel, U.-P. Influence of the Fe: Ni Ratio in FexNi9-xS8 (x = 3–6) on the CO2 Electroreduction. ChemElectroChem 2021, 8, 3161–3167. [Google Scholar] [CrossRef]
- Junge Puring, K.; Siegmund, D.; Timm, J.; Möllenbruck, F.; Schemme, S.; Marschall, R.; Apfel, U.-P. Electrochemical CO2 Reduction: Tailoring Catalyst Layers in Gas Diffusion Electrodes. Adv. Sustain. Syst. 2021, 5, 2000088. [Google Scholar] [CrossRef]
- Hoof, L.; Thissen, N.; Pellumbi, K.; Junge Puring, K.; Siegmund, D.; Mechler, A.K.; Apfel, U.-P. Hidden parameters for electrochemical carbon dioxide reduction in zero-gap electrolyzers. Cell Rep. Phys. Sci. 2022, 3, 100825. [Google Scholar] [CrossRef]






| Compound | λmax/nm (log ε) |
|---|---|
| L1 | 201 (4.65), 226 (4.71), 297 (3.95), 308 (3.95) |
| L2 | 204 (4.99), 229 (4.97), 272 (3.94), 308 (3.93), 409 (2.96) |
| L3a | 245 (5.28), 270 (4.92), 280 (4.97), 348 (4.91), 384 (4.35), 484 (4.04) |
| L4 | 212 (4.64), 229 (4.83), 291 (4.08), 424 (3.63) |
| L5 | 208 (4.91), 228 (4.94), 300 (3.94), 429 (3.43) |
| L6 | 208 (4.82), 229 (4.88), 265 (4.63), 306 (4.27), 317 (4.25), 517 (4.09) |
| Ag1b | 229 (5.35) 297 (4.58) |
| Ag2 | 204 (5.20), 229 (5.19), 308 (4.23), 412 (3.30) |
| Ag3 | 239 (5.35), 269 (5.02), 279 (5.06), 347 (5.01), 383 (4.45), 474 (4.11) |
| Ag4 | 212 (4.92), 229 (5.12), 292 (4.36), 422 (3.88) |
| Ag5 | 207 (5.12), 228 (5.17), 300 (4.22), 429 (3.72) |
| Ag6 | 206 (5.05), 230 (5.09), 268 (4.86), 308 (4.51), 537 (4.38) |
| Ered | Eox | |||||
|---|---|---|---|---|---|---|
| Compound | /V | /V | /V | /V | /V | /V |
| L4 | −1.20 | −0.79 | −1.78 | - | 1.25 | - |
| L6 | −1.27 | −0.94 | −1.74 | - | 0.69 | 0.64 |
| [Ag(ACN)4]BF4 | - | - | - | 0.80 | - | - |
| Ag1a | −0.82 | −0.72 | −0.99 | - | - | - |
| Ag2 | −1.32 | −1.08 | −1.82 | 0.67 | - | - |
| Ag3 | −0.98 | −0.89 | −1.83 | 0.70 | 1.30 | - |
| Ag4 | −1.20 | −0.84 | −1.82 | 0.53 | 1.32 | - |
| Ag5 | −1.11 | −0.66 | −1.76 | 0.52 | 1.35 | - |
| Ag6 | −1.26 | −0.93 | −1.73 | 0.50 | 0.81 | 0.71 |
| ACN | ACN + 2% H2O | |||
|---|---|---|---|---|
| Compound | jAr/µA cm−2 | /µA cm−2 | jAr/µA cm−2 | µA cm−2 |
| L6 | −65 | −198 | −81 | −205 |
| Ag1a | −118 | −187 | −149 | −244 |
| Ag2b | −103 | −297 | −116 | −513 |
| Ag3 | −180 | −156 | −255 | −700 |
| Ag4 | −177 | −354 | −187 | −895 |
| Ag5 | −166 | −488 | −149 | −1263 |
| Ag6 | −159 | −2264 | −158 | −2058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisch, D.; Sun, H.; Pellumbi, K.; Faust, K.; Apfel, U.-P.; Schöfberger, W. Tuning the Electronic Properties of Homoleptic Silver(I) bis-BIAN Complexes towards Efficient Electrocatalytic CO2 Reduction. Catalysts 2022, 12, 545. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050545
Krisch D, Sun H, Pellumbi K, Faust K, Apfel U-P, Schöfberger W. Tuning the Electronic Properties of Homoleptic Silver(I) bis-BIAN Complexes towards Efficient Electrocatalytic CO2 Reduction. Catalysts. 2022; 12(5):545. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050545
Chicago/Turabian StyleKrisch, Dominik, He Sun, Kevinjeorjios Pellumbi, Kirill Faust, Ulf-Peter Apfel, and Wolfgang Schöfberger. 2022. "Tuning the Electronic Properties of Homoleptic Silver(I) bis-BIAN Complexes towards Efficient Electrocatalytic CO2 Reduction" Catalysts 12, no. 5: 545. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050545