Comparative Catalytic Performance Study of 12-Tungstophosphoric Heteropoly Acid Supported on Mesoporous Supports for Biodiesel Production from Unrefined Green Seed Canola Oil
Abstract
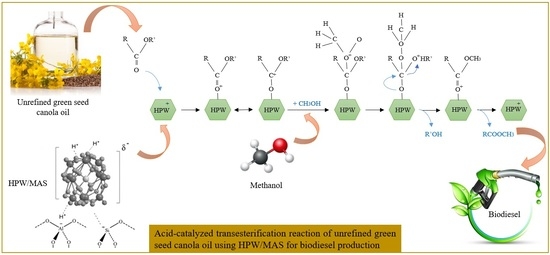
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Mesoporous Aluminosilicate (MAS)
2.3. Synthesis of Mesoporous Aluminophosphate (MAP)
2.4. Impregnation of 12-Tungstophosphoric Heteropoly Acid (HPW) on Supports
2.5. Catalytic Reaction
2.6. Catalyst Characterization Techniques
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalytic Activity
3.3. Reusability Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| BJH | Barrett–Joyner–Halenda |
| CTAB | Cetyltrimethylammonium bromide |
| FAAE | Fatty acid alkyl ester |
| FFAs | Free fatty acids |
| HPAs | Heteropoly acids |
| HPW | 12-tungstophosphoric heteropoly acid |
| HPW/MAP | Mesoporous aluminophosphate supported 12-tungstophosphoric heteropoly acid |
| HPW/MAS | Mesoporous aluminosilicate supported 12-tungstophosphoric heteropoly acid |
| HPW/γ-Al2O3 | Gamma alumina supported 12-tungstophosphoric heteropoly acid |
| MAP | Mesoporous aluminophosphate |
| MAS | Mesoporous aluminosilicate |
| NH3-TPD | Temperature-programmed desorption of ammonia |
| TEOS | Tetraethylorthosilicate |
| TMAOH | Tetramethyl ammonium hydroxide |
| TPAOH | Tetrapropyl ammonium hydroxide |
References
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Abdul Sattar, M. Renewable energy in the 21st century: A review. Mater. Today Proc. 2021, 5–8, In Press. [Google Scholar] [CrossRef]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A.K. Kinetics and Selectivity Study of Fischer-Tropsch Synthesis to c5+ Hydrocarbons: A Review; MDPI: Basel, Switzerland, 2021; Volume 11, ISBN 1306966477. [Google Scholar]
- Silvestre, W.P.; Pauletti, G.F.; Baldasso, C. Fodder radish (Raphanus sativus L.) seed cake as a feedstock for pyrolysis. Ind. Crops Prod. 2020, 154, 112689. [Google Scholar] [CrossRef]
- Anish Kumar, K.; Senthil Kumar, P.; Madhusudanan, S.; Pasupathy, V.; Vignesh, P.R.; Sankaranarayanan, A.R. A simplified model for evaluating best biodiesel production method: Fuzzy analytic hierarchy process approach. Sustain. Mater. Technol. 2017, 12, 18–22. [Google Scholar] [CrossRef]
- Orugba, H.O.; Edomwonyi-Otu, L.C. Improving the activity and stability of turtle shell-derived catalyst in alcoholysis of degraded vegetable oil: An experimental design approach. J. King Saud Univ. Eng. Sci. 2021; In Press. [Google Scholar]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Wong, K.Y.; Ng, J.H.; Chong, C.T.; Lam, S.S.; Chong, W.T. Biodiesel process intensification through catalytic enhancement and emerging reactor designs: A critical review. Renew. Sustain. Energy Rev. 2019, 116, 109399. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; Van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef] [Green Version]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Yabushita, M.; Nishitoba, T.; Osuga, R.; Yoshida, M.; Matsubara, M.; Maki, S.; Kanie, K.; Yokoi, T.; Cao, W.; et al. Organic Structure-Directing Agent-Free Synthesis of Mordenite-Type Zeolites Driven by Al-Rich Amorphous Aluminosilicates. ACS Omega 2021, 6, 5176–5182. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Harvey, A.P.; Thanapimmetha, A.; Srinophakun, P. Kinetic of myristic acid esterification with methanol in the presence of triglycerides over sulfated zirconia. Renew. Energy 2011, 36, 2679–2686. [Google Scholar] [CrossRef]
- Niu, M.; Kong, X. Efficient biodiesel production from waste cooking oil using p-toluenesulfonic acid doped polyaniline as a catalyst. RSC Adv. 2015, 5, 27273–27277. [Google Scholar] [CrossRef]
- Surasit, C.; Yoosuk, B.; Pohmakotr, M.; Tantirungrotechai, J. Biodiesel Synthesis from Palm Fatty Acid Distillate Using Tungstophosphoric Acid Supported on Cesium-Containing Niobia. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 465–474. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai, C.T.Q.; Ng, F.T.T. A kinetic model for a single step biodiesel production from a high free fatty acid (FFA) biodiesel feedstock over a solid heteropolyacid catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Negm, N.A.; Sayed, G.H.; Yehia, F.Z.; Habib, O.I.; Mohamed, E.A. Biodiesel production from one-step heterogeneous catalyzed process of Castor oil and Jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. J. Mol. Liq. 2017, 234, 157–163. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effects of support modifiers on the catalytic performance of Ni/Al2O3 catalyst in CO2 reforming of methane. Fuel 2014, 129, 197–203. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Solid Acid Catalysts for Biodiesel Production. Comments Inorg. Chem. 2020, 40, 176–216. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Sorokina, K.N.; Samoylova, Y.V.; Rodikova, Y.A.; Parmon, V.N. Direct Conversion of Microalgae Biomass to Formic Acid under an Air Atmosphere with Soluble and Solid Mo-V-P Heteropoly Acid Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 18947–18956. [Google Scholar] [CrossRef]
- Baroi, C.; Dalai, A.K. Review on biodiesel production from various feedstocks using 12-tungstophosphoric acid (TPA) as a solid acid catalyst precursor. Ind. Eng. Chem. Res. 2014, 53, 18611–18624. [Google Scholar] [CrossRef]
- Esmi, F.; Borugadda, V.B.; Dalai, A.K. Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: A Review. Catal. Today, 2022; In Press. [Google Scholar]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Gong, S.W.; Lu, J.; Wang, H.H.; Liu, L.J.; Zhang, Q. Biodiesel production via esterification of oleic acid catalyzed by picolinic acid modified 12-tungstophosphoric acid. Appl. Energy 2014, 134, 283–289. [Google Scholar] [CrossRef]
- Srilatha, K.; Lingaiah, N.; Sai Prasad, P.S.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N. Kinetics of the esterification of palmitic acid with methanol catalyzed by 12-tungstophosphoric acid supported on ZrO2. React. Kinet. Mech. Catal. 2011, 104, 211–226. [Google Scholar] [CrossRef]
- Kumbar, S.M.; Shanbhag, G.V.; Lefebvre, F.; Halligudi, S.B. Heteropoly acid supported on titania as solid acid catalyst in alkylation of p-cresol with tert-butanol. J. Mol. Catal. A Chem. 2006, 256, 324–334. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Wen, J.; Chen, A. One-step synthesis of mesoporous H4SiW12O40-SiO2 catalysts for the production of methyl and ethyl levulinate biodiesel. Catal. Commun. 2013, 34, 58–63. [Google Scholar] [CrossRef]
- Pires, L.H.O.; de Oliveira, A.N.; Monteiro, O.V., Jr.; Angélica, R.S.; da Costa, C.E.F.; Zamian, J.R.; do Nascimento, L.A.S.; Filho, G.N.R. Esterification of a waste produced from the palm oil industry over 12-tungstophosforic acid supported on kaolin waste and mesoporous materials. Appl. Catal. B Environ. 2014, 160–161, 122–128. [Google Scholar] [CrossRef]
- Kurhade, A.; Zhu, J.; Hu, Y.; Dalai, A.K. Surface Investigation of Tungstophosphoric Acid Supported on Ordered Mesoporous Aluminosilicates for Biodiesel Synthesis. ACS Omega 2018, 3, 14064–14075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Hoo, P.Y.; Abdullah, A.Z. Direct synthesis of mesoporous 12-tungstophosphoric acid SBA-15 catalyst for selective esterification of glycerol and lauric acid to monolaurate. Chem. Eng. J. 2014, 250, 274–287. [Google Scholar] [CrossRef]
- Kurhade, A.; Zhu, J.; Dalai, A.K.; Zhu, J.; Hu, Y.; Dalai, A.K. Meso-Structured HPW-MAS-7 and HPW-MAS-9 Composite Catalysts for Biodiesel Synthesis from Unre fi ned Green Seed Canola Oil. Fuel Process. Technol. 2018, 3, 14064–14075. [Google Scholar]
- Sudhakar, P.; Pandurangan, A. Heteropolyacid (H3PW12O40)-impregnated mesoporous KIT-6 catalyst for green synthesis of bio-diesel using transesterification of non-edible neem oil. Mater. Renew. Sustain. Energy 2019, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Kurhade, A.; Dalai, A.K. Physiochemical characterization and support interaction of alumina-supported heteropolyacid catalyst for biodiesel production. Asia-Pacific J. Chem. Eng. 2018, 13, e2249. [Google Scholar] [CrossRef]
- Tayebee, R.; Amini, M.M.; Akbari, M.; Aliakbari, A. A novel inorganic-organic nanohybrid material H4SiW12O40/pyridino-MCM-41 as efficient catalyst for the preparation of 1-amidoalkyl-2-naphthols under solvent-free conditions. Dalt. Trans. 2015, 44, 9596–9609. [Google Scholar] [CrossRef] [PubMed]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T. Ultrasound-assisted transesterification of crude Jatropha oil using alumina-supported heteropolyacid catalyst. Appl. Energy 2013, 105, 380–388. [Google Scholar] [CrossRef]
- Sawant, D.P.; Vinu, A.; Jacob, N.E.; Lefebvre, F.; Halligudi, S.B. Formation of nanosized zirconia-supported 12-tungstophosphoric acid in mesoporous silica SBA-15: A stable and versatile solid acid catalyst for benzylation of phenol. J. Catal. 2005, 235, 341–352. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. 12-Tungstophosphoric acid anchored to SBA-15: An efficient, environmentally benign reusable catalysts for biodiesel production by esterification of free fatty acids. Appl. Catal. A Gen. 2011, 403, 161–172. [Google Scholar] [CrossRef]
- Madhusudhan Rao, P.; Wolfson, A.; Kababya, S.; Vega, S.; Landau, M.V. Immobilization of molecular H3PW12O40 heteropolyacid catalyst in alumina-grafted silica-gel and mesostructured SBA-15 silica matrices. J. Catal. 2005, 232, 210–225. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Narkhede, N.; Brahmkhatri, V.; Patel, A. Efficient synthesis of biodiesel from waste cooking oil using solid acid catalyst comprising 12-tungstosilicic acid and SBA-15. Fuel 2014, 135, 253–261. [Google Scholar] [CrossRef]
- Patel, A.; Narkhede, N. 12-tungstophosphoric acid anchored to zeolite Hβ: Synthesis, characterization, and biodiesel production by esterification of oleic acid with methanol. Energy and Fuels 2012, 26, 6025–6032. [Google Scholar] [CrossRef]
- Srilatha, K.; Ramesh Kumar, C.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N.; Sai Prasad, P.S.; Lingaiah, N. Efficient solid acid catalysts for esterification of free fatty acids with methanol for the production of biodiesel. Catal. Sci. Technol. 2011, 1, 662–668. [Google Scholar] [CrossRef]
- Fan, M.; Si, Z.; Sun, W.; Zhang, P. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. Sci. Eng. 2017, 59, 165–188. [Google Scholar] [CrossRef]










| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 7 | 100 | 0 |
| 15 | 0 | 100 |
| 28 | 0 | 100 |
| Catalyst | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) | ICP-OES W (wt.%) |
|---|---|---|---|---|
| γ-Al2O3 | 297 | 0.82 | 7.5 | - |
| HPW/γ-Al2O3 | 195 | 0.50 | 7.2 | 23.2 |
| MAP | 581 | 0.14 | 4.7 | - |
| HPW/MAP | 111 | 0.08 | 5.1 | 22.5 |
| MAS | 703 ± 9 | 0.99 ± 0.03 | 5.0 ± 0.7 | - |
| HPW/MAS | 504 | 0.68 | 4.4 | 22.8 |
| Catalyst | Total Acidity (µmol/g) | 100–300 °C | 300–500 °C | 500–600 °C |
|---|---|---|---|---|
| γ-Al2O3 | 922 | 354 | 429 | 139 |
| HPW/γ-Al2O3 | 913 | 328 | 480 | 105 |
| R *-HPW/γ-Al2O3 | 876 | 332 | 453 | 91 |
| MAP | 795 ± 8 | 283 | 220 | 292 |
| HPW/MAP | 841 | 245 | 348 | 248 |
| R *-HPW/MAP | 835 | 292 | 302 | 241 |
| MAS | 1015 ± 16 | 270 | 276 | 469 |
| HPW/MAS | 1111 ± 13 | 466 | 448 | 196 |
| R *-HPW/MAS | 1082 | 449 | 565 | 68 |
| Catalyst | Reaction Conditions | Conversion or Yield (wt.%) | Ref. |
|---|---|---|---|
| HPA/MK700 | Temperature = 200 °C, reaction time = 2 h, alcohol to oil molar ratio = 10, catalyst loading = 10 wt.% | Conversion = 83.0 | [29] |
| H4SiW12O40-SiO2 | Temperature = 65 °C, reaction time = 6 h, catalyst loading = 20 wt.% | Yield = 73.0 | [28] |
| TPA/SBA-15 | Temperature = 65 °C, reaction time = 8 h, alcohol to oil molar ratio = 8, catalyst loading = 0.3 g | Conversion = 86.0 | [42] |
| TPA/zeolite Hβ | Temperature = 60 °C, reaction time = 6 h, alcohol to oil molar ratio = 20, catalyst loading = 30 wt.% | Conversion = 84.0 | [43] |
| TPA/SnO2 | Temperature = 65 °C, reaction time = 200 min, alcohol to oil molar ratio = 14, catalyst loading = 1 g | Conversion = 81.2 | [44] |
| HPW/γ-Al2O3 | Temperature = 200 °C, reaction time = 7 h, alcohol to oil molar ratio = 20, catalyst loading = 3 wt.% | Yield = 64.2 | This study |
| HPW/MAP | Temperature = 200 °C, reaction time = 7 h, alcohol to oil molar ratio = 20, catalyst loading = 3 wt.% | Yield = 78.3 | This study |
| HPW/MAS | Temperature = 200 °C, reaction time = 7 h, alcohol to oil molar ratio = 20, catalyst loading = 3 wt.% | Yield = 82.3 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmi, F.; Masoumi, S.; Dalai, A.K. Comparative Catalytic Performance Study of 12-Tungstophosphoric Heteropoly Acid Supported on Mesoporous Supports for Biodiesel Production from Unrefined Green Seed Canola Oil. Catalysts 2022, 12, 658. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12060658
Esmi F, Masoumi S, Dalai AK. Comparative Catalytic Performance Study of 12-Tungstophosphoric Heteropoly Acid Supported on Mesoporous Supports for Biodiesel Production from Unrefined Green Seed Canola Oil. Catalysts. 2022; 12(6):658. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12060658
Chicago/Turabian StyleEsmi, Fahimeh, Shima Masoumi, and Ajay K. Dalai. 2022. "Comparative Catalytic Performance Study of 12-Tungstophosphoric Heteropoly Acid Supported on Mesoporous Supports for Biodiesel Production from Unrefined Green Seed Canola Oil" Catalysts 12, no. 6: 658. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12060658