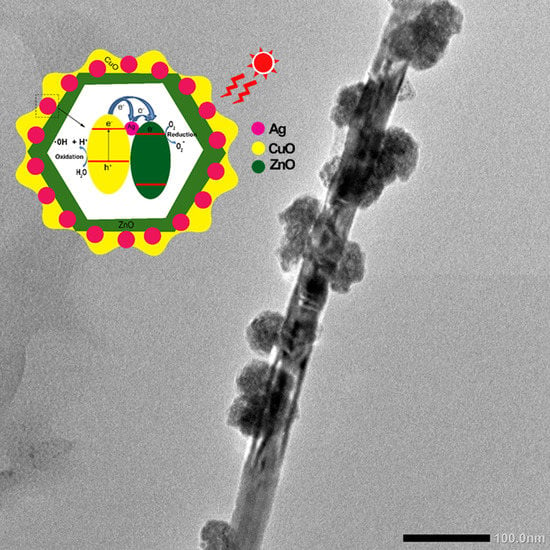
Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZnO Nanowires
3.3. Synthesis of Ag-ZnO and CuO-Ag-ZnO Nanowires
3.4. Sample Characterization
3.5. Photoelectrochemical Experiments
3.6. Photocatalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Dandeneau, C.S.; Zhou, Z.; Cao, G. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2019, 21, 4087–4108. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Xu, K.; Wu, J.; Tan, C.F.; Ho, G.W.; Wei, A.; Hong, M. Ag-CuO-ZnO metal-semiconductor multiconcentric nanotubes for achieving superior and perdurable photodegradation. Nanoscale 2017, 9, 11574–11583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Li, Y.; Lin, X.; Wang, Z.; Li, Z.; Wang, H.; Zhu, L. Preparation and photoelectrocatalytic performance of Fe2O3/ZnO composite electrode loading on conductive glass. Chem. J. Chin. Univ. 2018, 39, 771–778. [Google Scholar]
- Han, Z.; Li, Y.; Chen, F.; Tang, S.; Wang, P. Preparation of ZnO/Ag2O nanofilbers by coaxial electrospinning and study of their photocatalytic properties. Chem. J. Chin. Univ. 2020, 41, 308–316. [Google Scholar]
- Pivet, M.L.; Poupart, R.; Capochichi-Gnambodoe, M.; Martin, N.; Leprince-Wang, Y. Direct growth of ZnO nanowires on civil engineering materials: Smart materials for supported photodegradation. Microsyst. Nanoeng. 2019, 5, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Li, S.; Wang, X.; Fang, F.; Chu, X.; Wei, Z.; Li, J.; Chen, X.; Wang, F. The growth and photocatalytic property of ZnO nanofibers synthesized by atom layer deposition using PVP nanofibers as templates. Appl. Surf. Sci. 2012, 263, 14–17. [Google Scholar]
- Mauro, A.D.; Zimbone, M.; Scuderi, M.; Nicotra, G.; Fragalà, M.E.; Impellizzeri, G. Effect of Pt nanoparticles on the photocatalytic activity of ZnO nanofibers. Nanoscale Res. Lett. 2015, 10, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Q.; Duan, X.; Ng, D.H.L.; Tang, H.; Yang, Y.; Kong, M.; Wu, Z.; Cai, W.; Wang, G. Ag nanoparticle decorated nanoporous ZnO microrods and their enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2012, 4, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In situ charge transfer at the Ag@ZnO photoelectrochemical interface toward the high photocatalytic performance of H2 evolution and RhB degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Thinh, V.D.; Lam, V.D.; Bach, T.N.; Van, N.D.; Manh, D.H.; Tung, D.H.; Lien, N.T.H.; Thuy, U.T.D.; Anh, T.X.; Tung, N.T.; et al. Enhanced optical and photocatalytic properties of Au/Ag nanoparticle-decorated ZnO films. J. Electron. Mate. 2020, 49, 2626–2632. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carrier and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+—TiO2 photocatalysts toward visible photooxidation for water and wastewater treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef]
- Moon, G.D.; Joo, J.B.; Lee, I.; Yin, Y. Decoration of size-tunable CuO nanodots on TiO2 nanocrystals for noble metal-free photocatalytic H2 production. Nanoscale 2014, 6, 12002–12008. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Shi, Q.; Ping, G.; Xu, H.; Waleed, A.; Li, G. CuO nanoparticle-decorated TiO2-nanotube heterojunctions for direct synthesis of methyl formate via photo-oxidation of methanol. ACS Omega 2020, 5, 15942–15948. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO decorated with CeO2 nanoparticles as the direct Z-scheme heterojunction for enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, Z.; Kang, L.; Li, M.; Gao, Z. The facile preparation of Ag decorated TiO2/ZnO nanotube and their potent photocatalytic degradation efficiency. RSC Adv. 2017, 7, 50064–50071. [Google Scholar] [CrossRef] [Green Version]
- Mulkhopadhyay, S.; Maiti, D.; Chatterjee, S.; Devi, P.S.; Kumar, G.S. Design and application of Au decorated ZnO/TiO2 as a stable photocatalyst for wide spectral coverage. Phys. Chem. Chem. Phys. 2016, 18, 31622–31633. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.C.; Gustavo, C.; Ana, B.; Ricardo, S.S. Synthesis and Characterization of a ZnO/CuO/Ag Composite and its Application as a Photocatalyst for Methyl Orange Degradation. Int. J. Electrochem. Sci. 2018, 13, 9242–9256. [Google Scholar]
- Tsai, C.-E.; Yeh, S.-M.; Chen, C.-H.; Lin, H.-N. Flexible photocatalytic paper with Cu2O and Ag nanoparticle-decorated ZnO nanorods for visible light photodegradation of organic dye. Nanoscale Res. Lett. 2019, 14, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, A.; Wang, B.; Zhang, H.; Ding, P.; Wang, Q. Sandwiched ZnO@Au@Cu2O Nanorod Films as Efficient Visible-Light-Driven Plasmonic Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 4066–4074. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. ZnS—Ag—ZnO as an excellent UV-light-active photocatalyst for the degradation of AV 7, AB 1, RR 120, and RY 84 dyes: Synthesis, characterization, and catalytic applications. Ind. Eng. Chem. Res. 2014, 53, 12953–12963. [Google Scholar] [CrossRef]
- Gavade, N.L.; Babar, S.B.; Kadam, A.N.; Gophane, A.; Garadka, K.M. Fabrication of M@CuxO/ZnO (M = Ag, Au) heterostructured nanocomposite with enhanced photocatalytic performance under sunlight. Ind. Eng. Chem. Res. 2017, 56, 14489–14501. [Google Scholar] [CrossRef]
- Kumar, S.; Saralch, S.; Pathak, D. Low-cost deposition of cupric oxide thin films for optoelectronic applications. Eur. Phys. J. Appl. Phys. 2018, 84, 20301. [Google Scholar]
- Lin, J.; Yao, C.; Wu, L.; Jiang, K.; Hu, Z.; Li, L.; Xu, N.; Sun, J.; Wu, J. CuO: Synthesis in a highly excited oxygen-copper plasma and decoration of ZnO nanorods for enhanced photocatalysis. J. Phys. Chem. C 2021, 125, 9119–9128. [Google Scholar] [CrossRef]
- Xu, H.; Fang, W.; Xu, L.; Liu, F. Batch preparation of CuO/ZnO-loaded nanofiber membranes for photocatalytic degradation of organic dyes. Langmuir 2020, 336, 14189–14202. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Mohammad, N.; Pourjavadi, A.; Mehdipour, H.; Moshfegh, A.Z. Tuning composition of electrospun ZnO/CuO nanofibers: Toward controllable and efficient solar photocatalytic degradation of organic pollutants. J. Phys. Chem. C 2017, 121, 3327–3338. [Google Scholar] [CrossRef]
- Sapkota, B.B.; Mishra, S.R. A simple ball milling method for the preparation of p-CuO/n-ZnO nanocomposite photocatalysts with high photocatalytic activity. J. Nanosci. Nanotechnol. 2013, 13, 6588–6596. [Google Scholar] [CrossRef]
- Yuan, X.; Pei, F.; Luo, X.; Hu, H.; Qian, H.; Wen, P.; Miao, K.; Guo, S.; Wang, W.; Feng, G. Fabrication of ZnO/Au@Cu2O heterojunction towards deeply oxidative photodegradation of organic dyes. Sep. Purif. Technol. 2021, 262, 118301. [Google Scholar] [CrossRef]
- Vlad, I.E.; Marisca, O.T.; Vulpoi, A.; Simon, S.; Leopold, N.; Anghel, S.D. Simple approach for gold nanoparticle synthesis using an Ar-bubbled plasma setup. J. Nanopart. Res. 2014, 16, 2633. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, Z.; Zeng, Z.; Liu, F.; Qin, J. Hydrothermal fabrication of hierarchical CuO nanoflowers for dual-function amperometric sensing of hydrogen peroxide and glucose. New J. Chem. 2019, 43, 18629–18636. [Google Scholar] [CrossRef]
- Pal, J.; Ganguly, M.; Dutta, S.; Mondal, C.; Negishi, Y.; Pal, T. Hierarchical Au-CuO Nanocomposite from Redox Transformation Reaction for Surface Enhanced Raman Scattering and Clock Reaction. CrystEngComm 2014, 16, 883–893. [Google Scholar] [CrossRef]
- Nishino, F.; Jeem, M.; Zhang, L.; Okamoto, K.; Okabe, S.; Watanabe, S. Formation of CuO nano-flowered surfaces via submerged photo-synthesis of crystallites and their antimicrobial activity. Sci. Rep. 2017, 7, 1063. [Google Scholar] [CrossRef]
- Mahardika, T.; Putri, N.A.; Putri, A.P.; Fauzia, V.; Roza, L.; Sugihartono, I.; Herbani, Y. Rapid and low temperature synthesis of Ag nanoparticles on the ZnO nanorods for photocatalytic activity improvement. Results Phys. 2019, 13, 102209. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p–n CuO–ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(vi). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-band ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Zhang, Z.; Wang, X.; Zhan, R.; Chen, H.; Deng, S.; Xu, N.; Chen, J. Mechanism of photoluminescence quenching in visible and ultraviolet emissions of ZnO nanowires decorated with gold nanoparticles. Jpn. J. Appl. Phys. 2019, 58, 051005. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kataria, J.; Bhardwaj, V.K.; Sharma, S. Green biomimetic preparation of efficient Ag–ZnO heterojunctions with excellent photocatalytic performance under solar light irradiation: A novel biogenic-deposition-precipitation approach. Nanoscale Adv. 2019, 1, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Y.; Jian, W.-J.; Dang, H.-F.; Zhao, X.-F.; Zhang, L.-Z.; Li, J.-H. Hierarchical Ag-ZnO Microspheres with Enhanced Photocatalytic Degradation Activities. Pol. J. Environ. Stud. 2017, 26, 871–880. [Google Scholar] [CrossRef]
- Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 29, 122983. [Google Scholar] [CrossRef]
- Zihan, Z.; Feng, G.; Zhonghao, X.; Xiaoxuan, D.; Quian, Z. Photocatalytic degradation of an organophosphorus pesticide using a ZnO/rGO composite. RSC Adv. 2020, 10, 11929–11938. [Google Scholar]
- Hongyu, M.; Caixia, S.; Yanhong, Z.; Bo, Z.; Debao, W. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar]
- Hu, W.; Zhang, Q.; Luo, K.; Yuan, H.; Li, J.; Xu, M.; Xu, S. Enhanced photocatalytic properties of CuO-ZnO nanocomposites by decoration with Ag nanoparticles. Ceram. Int. 2020, 46, 24753–24757. [Google Scholar] [CrossRef]
- Emily, A.; Emma, K.A.; Eftihia, B.; Jonathon, A.B. CuO enhances the photocatalytic activity of Fe2O3 through synergistic reactive oxygen species interactions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125179–125202. [Google Scholar]
- Ren, S.; Zhao, G.; Wang, Y.; Wang, B.; Wang, Q. Enhanced photocatalytic performance of sandwiched ZnO@Ag@Cu2O nanorod films: The distinct role of Ag NPs in the visible light and UV region. Nanotechnology 2015, 26, 125403. [Google Scholar] [CrossRef]











| Sr. No. | Catalyst Materials | Synthesis Routes | Synthesis Time | Light Source | * k | Ref. |
|---|---|---|---|---|---|---|
| 01 | Ag–CuO–ZnO NRs | Sol-gel method Photodeposition (Ag, CuO) | 5 h 4 h | Xenon lamp (50 mW cm−2, 420–720 nm) | ∼0.1 min−1 | [5] |
| 02 | Ag–CuO–ZnO NTs | Sol-gel method Photodeposition (Ag, CuO) | 5 h 4 h | Xenon lamp (50 mW cm−2, 420–720 nm) | ∼0.3 min−1 | [5] |
| 03 | ZnO@Au@Cu2O | Electrochemical method Sputtering method (Au, Cu2O) | 1 h 60 s | Solar simulator (Bos-X350-Z, -AM, 60 mW/cm2) | 0.3 h−1 | [24] |
| 04 | Ag@CuxO/ZnO | Milling method Reduction method (Ag, Cu2O) | 4 h 3 h | UV (365 nm) | 18.06 × 10−2 min−1 | [26] |
| 05 | CuO-ZnO-Ag | Sol-gel method | - | UV-VIS | 0.0885 min−1 | [47] |
| 06 | (ZnO)0.2/(Fe2O3)0.6/(CuO)0.2 | Hydrothermal (ZnO) Precipitation method (Fe2O3) Hydrothermal (CuO) | 1.5 h 2 weeks 1.5 h | Two tungsten halogen lamps (ASI Illuminator, 50 W each) | 0.039 min−1 | [48] |
| 07 | ZnO@Ag@Cu2O | Electrochemical method Sputtering method (Au, Cu2O) | 1 h 60 s | Solar simulator (Bos-X350-Z, -AM, 60 mW/cm2) | 0.3 h−1 | [49] |
| 08 | CuO-Ag-ZnO Present work | Hydrothermal (ZnO) Plasma electrolytic oxidation (PEO) treatment (CuO, Ag) | 3 h 10 min. | Solar simulator (Oriel Sol1A, 100 mW cm−2) | 0.2007 min−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, P.T.; Thinh, V.D.; Lam, V.D.; Bach, T.N.; Phong, L.T.H.; Tung, D.H.; Manh, D.H.; Van Khien, N.; Anh, T.X.; Le, N.T.H. Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency. Catalysts 2022, 12, 801. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070801
Thu PT, Thinh VD, Lam VD, Bach TN, Phong LTH, Tung DH, Manh DH, Van Khien N, Anh TX, Le NTH. Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency. Catalysts. 2022; 12(7):801. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070801
Chicago/Turabian StyleThu, Phung Thi, Vu Duy Thinh, Vu Dinh Lam, Ta Ngoc Bach, Le Thi Hong Phong, Do Hoang Tung, Do Hung Manh, Nguyen Van Khien, Trinh Xuan Anh, and Ngo Thi Hong Le. 2022. "Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency" Catalysts 12, no. 7: 801. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070801