2D Graphene Sheets as a Sensing Material for the Electroanalysis of Zileuton
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Sensing Material
2.2. Electrochemical Characterization of the Fabricated Electrodes
2.3. Cyclic Voltammetric Investigation of ZLT
2.4. Impact of the Immersion Time
2.5. Effect of PBS
2.6. Impact of Scan Rate Variation
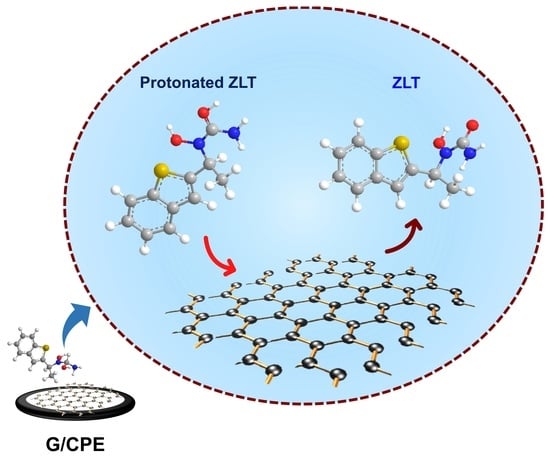
2.7. The Probable Electrochemical Sensing and Reaction Mechanism
2.7.1. Electrode Sensing Mechanism
2.7.2. Probable Electrochemical Reaction Mechanism
2.8. Quantification of ZLT
2.9. ZLT Determination in Spiked Urine Samples
2.10. Study of Interference
2.11. Stability of the Electrode
3. Materials and Methods
3.1. Used Chemicals
3.2. Equipment and Instruments Used
3.3. Construction of the Working Sensor
3.4. Method of Analysis
3.5. Test Solution for Real Sample and Interference Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gener, P.; Montero, S.; Xandri-Monje, H.; Díaz-Riascos, Z.V.; Rafael, D.; Andrade, F.; Martínez-Trucharte, F.; González, P.; Seras-Franzoso, J.; Manzano, A. Zileuton™ loaded in polymer micelles effectively reduce breast cancer circulating tumor cells and intratumoral cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102106. [Google Scholar] [CrossRef]
- Muthukrishnan, P.T.; Nouraie, M.; Parikh, A.; Holguin, F. Zileuton use and phenotypic features in asthma. Pulm. Pharmacol. Ther. 2020, 60, 101872. [Google Scholar] [CrossRef]
- Dahlin, A.; Qiu, W.; Litonjua, A.A.; Lima, J.J.; Tamari, M.; Kubo, M.; Irvin, C.G.; Peters, S.P.; Wu, A.C.; Weiss, S.T. The phosphatidylinositide 3-kinase (PI3K) signaling pathway is a determinant of zileuton response in adults with asthma. Pharm. J. 2018, 18, 665–677. [Google Scholar] [CrossRef]
- Goode, J.-V.R.; Small, R.E. Leukotriene modifiers. J. Am. Pharm. Assoc. (1996) 1998, 38, 48–57. [Google Scholar] [CrossRef]
- Berger, W.; De Chandt, M.; Cairns, C. Zileuton: Clinical implications of 5-Lipoxygenase inhibition in severe airway disease. Int. J. Clin. Pract. 2007, 61, 663–676. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Krishnan, R.G.; Saraswathyamma, B. Disposable electrochemical sensor for coumarin induced milk toxicity in raw milk samples. Measurement 2021, 170, 108709. [Google Scholar] [CrossRef]
- Padmanaban, A.; Murugadoss, G.; Venkatesh, N.; Hazra, S.; Kumar, M.R.; Tamilselvi, R.; Sakthivel, P. Electrochemical determination of harmful catechol and rapid decolorization of textile dyes using ceria and tin doped ZnO nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105976. [Google Scholar] [CrossRef]
- Alizadeh, M.; Demir, E.; Aydogdu, N.; Zare, N.; Karimi, F.; Kandomal, S.M.; Rokni, H.; Ghasemi, Y. Recent advantages in electrochemical monitoring for the analysis of amaranth and carminic acid food colors. Food Chem. Toxicol. 2022, 163, 112929. [Google Scholar] [CrossRef]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuators B Chem. 2021, 345, 130344. [Google Scholar] [CrossRef]
- Killedar, L.; Ilager, D.; Malode, S.J.; Shetti, N.P. Fast and facile electrochemical detection and determination of fungicide carbendazim at titanium dioxide designed carbon-based sensor. Mater. Chem. Phys. 2022, 285, 126131. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Kalanur, S.S.; Pollet, B.G.; Nadagouda, M.N.; Aminabhavi, T.M. Hafnium doped tungsten oxide intercalated carbon matrix for electrochemical detection of perfluorooctanoic acid. Chem. Eng. J. 2022, 434, 134700. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Z.; Yang, S.; Rich, J.; Zhao, S.; Klingeborn, M.; Huang, P.-H.; Li, Z.; Stout, A.; Murphy, Q. Electrochemical micro-aptasensors for exosome detection based on hybridization chain reaction amplification. Microsyst. Nanoeng. 2021, 7, 63. [Google Scholar] [CrossRef]
- Gao, M.; Pan, S.-Y.; Chen, W.-C.; Chiang, P.-C. A cross-disciplinary overview of naturally derived materials for electrochemical energy storage. Mater. Today Energy 2018, 7, 58–79. [Google Scholar] [CrossRef]
- Killedar, L.S.; Shanbhag, M.M.; Shetti, N.P.; Malode, S.J.; Veerapur, R.S.; Reddy, K.R. Novel graphene-nanoclay hybrid electrodes for electrochemical determination of theophylline. Microchem. J. 2021, 165, 106115. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Bhakta, A.K.; Mekhalif, Z. Nano-graphene-platelet/Brilliant-green composite coated carbon paste electrode interface for electrocatalytic oxidation of flavanone Hesperidin. Microchem. J. 2021, 160, 105768. [Google Scholar] [CrossRef]
- Malode, S.J.; Keerthi, P.K.; Shetti, N.P.; Kulkarni, R.M. Electroanalysis of carbendazim using MWCNT/Ca-ZnO modified electrode. Electroanalysis 2020, 32, 1590–1599. [Google Scholar] [CrossRef]
- Yan, D.; Lou, Y.; Yang, Y.; Chen, Z.; Cai, Y.; Guo, Z.; Zhan, H.; Chen, B. Dye-modified metal–organic framework as a recyclable luminescent sensor for nicotine determination in urine solution and living cell. ACS Appl. Mater. Interfaces 2019, 11, 47253–47258. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Ilager, D.; Mahapatra, S.; Shetti, N.P.; Chandra, P. Amberlite XAD-4 based electrochemical sensor for diclofenac detection in urine and commercial tablets. Mater. Chem. Phys. 2021, 273, 125044. [Google Scholar] [CrossRef]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Singh, V.; Reddy, K.R.; Shetti, N.P.; Venkata Reddy, C.; Sadhu, V.; Naveen, S. Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Heerema, S.J.; Dekker, C. Graphene nanodevices for DNA sequencing. Nat. Nanotechnol. 2016, 11, 127–136. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Liu, Y.; Su, Z. Developing Graphene-Based Nanohybrids for Electrochemical Sensing. Chem. Rec. 2019, 19, 534–549. [Google Scholar] [CrossRef]
- Lin, D.; Su, Z.; Wei, G. Three-dimensional porous reduced graphene oxide decorated with MoS2 quantum dots for electrochemical determination of hydrogen peroxide. Mater. Today Chem. 2018, 7, 76–83. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Knezevic, N.Z. Graphene-based biosensors for on-site detection of contaminants in food. Anal. Methods 2018, 10, 5061–5070. [Google Scholar] [CrossRef]
- Novikov, S.; Lebedeva, N.; Satrapinski, A.; Walden, J.; Davydov, V.; Lebedev, A. Graphene based sensor for environmental monitoring of NO2. Sens. Actuators B Chem. 2016, 236, 1054–1060. [Google Scholar] [CrossRef]
- Kashif, M.; Jaafar, E.; Bhadja, P.; Low, F.W.; Sahari, S.K.; Hussain, S.; Loong, F.K.; Ahmad, A.; AlGarni, T.S.; Shafa, M. Effect of potassium permanganate on morphological, structural and electro-optical properties of graphene oxide thin films. Arab. J. Chem. 2021, 14, 102953. [Google Scholar] [CrossRef]
- Kashif, M.; Jafaar, E.; Sahari, S.K.; Low, F.W.; Hoa, N.D.; Ahmad, A.; Abbas, A.; Ngaini, Z.; Shafa, M.; Qurashi, A. Organic sensitization of graphene oxide and reduced graphene oxide thin films for photovoltaic applications. Int. J. Energy Res. 2021, 45, 9657–9666. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bagihalli, G.B.; Reddy, K.R.; Ravindranadh, K.; Reddy, C.V. A novel biosensor based on graphene oxide-nanoclay hybrid electrode for the detection of Theophylline for healthcare applications. Microchem. J. 2019, 149, 103985. [Google Scholar] [CrossRef]
- Bard, A.; Faulkner, L.; Leddy, J.; Zoski, C. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2, p. 231. [Google Scholar]
- Killedar, L.S.; Shanbhag, M.M.; Malode, S.J.; Bagihalli, G.B.; Mahapatra, S.; Mascarenhas, R.J.; Shetti, N.P.; Chandra, P. Ultra-sensitive detection of tizanidine in commercial tablets and urine samples using zinc oxide coated glassy carbon electrode. Microchem. J. 2022, 172, 106956. [Google Scholar] [CrossRef]
- Westbroek, P.; Priniotakis, G.; Kiekens, P. Analytical Electrochemistry in Textiles; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Arvand, M.; Ardaki, M.S.; Zanjanchi, M.A. A new sensing platform based on electrospun copper oxide/ionic liquid nanocomposite for selective determination of risperidone. RSC Adv. 2015, 5, 40578–40587. [Google Scholar] [CrossRef]
- Yin, T.; Li, H.; Su, L.; Liu, S.; Yuan, C.; Fu, D. The catalytic effect of TiO2 nanosheets on extracellular electron transfer of Shewanella loihica PV-4. Phys. Chem. Chem. Phys. 2016, 18, 29871–29878. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Satpati, A.K.; Basavaraja, B.M. A novel ZnO/reduced graphene oxide and Prussian blue modified carbon paste electrode for the sensitive determination of Rutin. Sci. China Chem. 2019, 62, 262–270. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Junkermeier, C.E.; Solenov, D.; Reinecke, T.L. Adsorption of NH2 on Graphene in the Presence of Defects and Adsorbates. J. Phys. Chem. C 2013, 117, 2793–2798. [Google Scholar] [CrossRef]
- Remko, M.; Lyne, P.D.; Richards, W.G. Molecular structure and gas-phase reactivity of zileuton and its N-dehydroxylated metabolite: Two-layered ONIOM calculations. Phys. Chem. Chem. Phys. 2000, 2, 2511–2514. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Kalanur, S.S.; Pollet, B.G.; Upadhyaya, K.P.; Ayachit, N.H.; Aminabhavi, T.M. Hf-Doped Tungsten Oxide Nanorods as Electrode Materials for Electrochemical Detection of Paracetamol and Salbutamol. ACS Appl. Nano Mater. 2021, 5, 1263–1275. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Shirkhedkar, A.A. Novel HPTLC and UV-AUC analyses: For simple, economical, and rapid determination of Zileuton racemate. Arab. J. Chem. 2017, 10, 360–367. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Dhumal, D.M.; Shirkhedkar, A.A. Development and validation of simple RP-HPLC-PDA analytical protocol for zileuton assisted with Design of Experiments for robustness determination. Arab. J. Chem. 2017, 10, 273–282. [Google Scholar] [CrossRef]
- Pian, P.; Labovitz, E.; Hoffman, K.; Clavijo, C.F.; Lynn, R.R.; Galinkin, J.L.; Vinks, A.A.; Malik, P.; Christians, U. Quantification of the 5-lipoxygenase inhibitor zileuton in human plasma using high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2013, 937, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Baezzat, M.R.; Banavand, F.; Fasihi, F. Highly sensitive determination of zileuton using TiO2 nanoparticles and the ionic liquid 1-hexylpyridinium hexafluorophosphate nanocomposite sensor. Ionics 2019, 25, 1835–1844. [Google Scholar] [CrossRef]
- Baezzat, M.R.; Bagheri, M.; Abdollahi, E. Molecularly imprinted polymer based sensor for measuring of zileuton: Evaluation as a modifier for carbon paste electrode in electrochemically recognition. Mater. Today Commun. 2019, 19, 23–31. [Google Scholar] [CrossRef]






| Methods/Sensors Utilized | LD (M) | Reference | |
|---|---|---|---|
| Determination of ZLT | UV-AUC analysis | 1.8 × 10−6 | [45] |
| Amperometry technique—TiO2/CILE | 1.8 × 10−6 | [48] | |
| RP-HPLC | 9.2 × 10−7 | [46] | |
| DPV technique—MIP/CPE | 8.0 × 10−7 | [49] | |
| HPLTC | 1.0 × 10−7 | [45] | |
| HPLC–MS | 1. 8 × 10−8 | [47] | |
| DPV technique—TiO2/CILE | 8.3 × 10−9 | [48] | |
| SWV technique—G/CPE | 3.3 × 10−8 | Present work |
| Drug Spiked | Urine Samples | Spiked (10−6 M) | Detected * (10−6 M) | % Recovery | RSD | % RSD |
|---|---|---|---|---|---|---|
| ZLT | Sample 1 | 1.0 | 0.932 | 93.20 | 0.026 | 2.61 |
| Sample 2 | 5.0 | 4.579 | 91.58 | 0.027 | 2.66 | |
| Sample 3 | 8.0 | 7.073 | 88.41 | 0.028 | 2.75 |
| Interferent | Detected Epa (V) | Standard Epa (V) | Change in % Epa |
|---|---|---|---|
| Excipients | |||
| Cellulose | 0.412 | 0.424 | −2.830 |
| Citric acid | 0.420 | 0.424 | −0.943 |
| Dextrose | 0.420 | 0.424 | −0.943 |
| Glycine | 0.416 | 0.424 | −1.887 |
| Starch | 0.416 | 0.424 | −1.887 |
| TiO2 | 0.432 | 0.424 | 1.887 |
| Metal Ions | |||
| CuSO4 | 0.420 | 0.424 | −0.943 |
| FeSO4 | 0.416 | 0.424 | −1.887 |
| KCl | 0.420 | 0.424 | −0.943 |
| KNO3 | 0.428 | 0.424 | 0.943 |
| MgCl2 | 0.428 | 0.424 | 0.943 |
| NaCl | 0.428 | 0.424 | 0.943 |
| (ZLT) = 1 × 10−5 M, (Excipient) = 1 × 10−3 M, (Metal Ions) = 1 × 10−3 M | |||
| Repeatability | |||||
|---|---|---|---|---|---|
| CV Responses | Detected Response (µA) | Original Response (µA) | Response Retention % | RSD | % RSD |
| At 1st day | 0.983 | 0.983 | 100.00 | 0.0183 | 1.83 |
| At 7th day | 0.965 | 0.983 | 98.17 | 0.0187 | 1.87 |
| At 14th day | 0.947 | 0.983 | 96.34 | 0.0190 | 1.90 |
| % Retention: 96.34–100.0; Average % retention: 98.16; % RSD: 1.86 | |||||
| Reproducibility | |||||
| At 0 h | 0.983 | 0.983 | 100.00 | 0.0219 | 2.19 |
| After 12 h | 1.025 | 0.983 | 104.27 | 0.0210 | 2.10 |
| After 24 h | 0.996 | 0.983 | 101.32 | 0.0216 | 2.16 |
| % Retention:100.0–104.27; Average % retention: 101.89; % RSD: 2.14 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanbhag, Y.M.; Shanbhag, M.M.; Malode, S.J.; Dhanalakshmi, S.; Mondal, K.; Shetti, N.P. 2D Graphene Sheets as a Sensing Material for the Electroanalysis of Zileuton. Catalysts 2022, 12, 867. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12080867
Shanbhag YM, Shanbhag MM, Malode SJ, Dhanalakshmi S, Mondal K, Shetti NP. 2D Graphene Sheets as a Sensing Material for the Electroanalysis of Zileuton. Catalysts. 2022; 12(8):867. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12080867
Chicago/Turabian StyleShanbhag, Yogesh M., Mahesh M. Shanbhag, Shweta J. Malode, S. Dhanalakshmi, Kunal Mondal, and Nagaraj P. Shetti. 2022. "2D Graphene Sheets as a Sensing Material for the Electroanalysis of Zileuton" Catalysts 12, no. 8: 867. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12080867