Catalytic Hydrodeoxygenation of Vanillin, a Bio-Oil Model Compound over Renewable Ni/Biochar Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
Physicochemical Properties of the Prepared Catalysts
2.2. Catalyst Activity
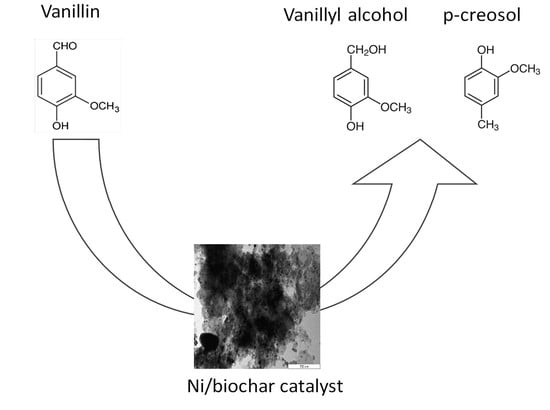
2.2.1. Conversion of Vanillin
2.2.2. Effect of Temperature on the HDO of Vanillin
2.2.3. Effect of Pressure on the HDO of Vanillin
2.3. Effect of Reaction Parameters
2.4. Catalyst Reusability
2.5. Effect of Chemical Treatment on Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Chemical Activation of Biochar
3.3. Acidification of Biochar
3.4. Preparation of Ni/Biochar Catalyst
3.5. Catalyst Characterization
3.6. Catalytic Reaction
3.7. Optimization of the Reaction Parameters over Ni/biochar Catalyst
3.8. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Mironenko, A.V.; Raizada, A.; Chen, T.; Mao, X.; Padmanabhan, A.; Vlachos, D.G.; Gorte, R.J.; Vohs, J.M. Mechanistic study of the direct hydrodeoxygenation of m-cresol over WO x-decorated Pt/C catalysts. ACS Catal. 2018, 8, 7749–7759. [Google Scholar] [CrossRef]
- Huang, R.; Kwon, O.; Lin, C.; Gorte, R.J. The effects of SMSI on m-Cresol hydrodeoxygenation over Pt/Nb2O5 and Pt/TiO2. J. Catal. 2021, 398, 102–108. [Google Scholar] [CrossRef]
- Aliu, E.; Hart, A.; Wood, J. Mild-Temperature hydrodeoxygenation of vanillin a typical bio-oil model compound to Creosol a potential future biofuel. Catal. Today 2021, 379, 70–79. [Google Scholar] [CrossRef]
- Aliu, E.; Hart, A.; Wood, J. Reaction kinetics of vanillin hydrodeoxygenation in acidic and nonacidic environments using bimetallic PdRh/Al2O3 catalyst. Energy Fuels 2019, 33, 11712–11723. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; Mäki-Arvela, P.; Wärnå, J.; Monzón, A.; Angel-Centeno, M.; Murzin, D.Y. HDO of vanillin over noble metal catalyst supported on biochars: Part II: Catalytic behaviour. Appl. Catal. B Environ. 2020, 268, 118425. [Google Scholar] [CrossRef]
- Neumann, J.; Meyer, J.; Ouadi, M.; Apfelbacher, A.; Binder, S.; Hornung, A. The conversion of anaerobic digestion waste into biofuels via a novel Thermo-Catalytic Reforming process. Waste Manag. 2016, 47, 141–148. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Shen, J.; Wang, X.S.; Li, C.-Z. Production and fuel properties of fast pyrolysis oil/bio-diesel blends. Fuel Process. Technol. 2010, 91, 296–305. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and application of biochar-based catalysts for biofuel production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts. RSC Adv. 2014, 4, 10731–10737. [Google Scholar] [CrossRef]
- Wang, B.; He, Z.; Zhang, B.; Duan, Y. Study on hydrothermal liquefaction of spirulina platensis using biochar based catalysts to produce bio-oil. Energy 2021, 230, 120733. [Google Scholar] [CrossRef]
- Yadav, K.; Jagadevan, S. Influence of process parameters on synthesis of biochar by pyrolysis of biomass: An alternative source of energy. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2019. [Google Scholar]
- Barik, D. Energy extraction from toxic waste originating from food processing industries. Energy Toxic Org. Waste Heat Power Gener. 2019, 3, 17–42. [Google Scholar] [CrossRef]
- Kohl, T.; Laukkanen, T.P.; Järvinen, M.P. Integration of biomass fast pyrolysis and precedent feedstock steam drying with a municipal combined heat and power plant. Biomass Bioenergy 2014, 71, 413–430. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature 2009, 458, 1158–1162. [Google Scholar] [CrossRef]
- Chen, T.; Kwon, O.; Huang, R.; Lin, C.; Vohs, J.M. WOx promoted nickel catalyst for hydrodeoxygenation of m-cresol. J. Catal. 2021, 400, 294–300. [Google Scholar] [CrossRef]
- Duan, M.; Cheng, Q.; Wang, M.; Wang, Y. In situ hydrodeoxygenation of vanillin over Ni–Co–P/HAP with formic acid as a hydrogen source. RSC Adv. 2021, 11, 10996–11003. [Google Scholar] [CrossRef]
- Cheng, Q.-y.; Liu, D.-j.; Wang, M.-m.; Wang, Y.-j. Study on catalytic performance of Ni-Co-P amorphous alloy for HDO of vanillin. J. Fuel Chem. Technol. 2019, 47, 1205–1213. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Sun, L.; Gao, S.; Gao, W.; Cheng, X.; Shang, N.; Gao, Y.; Wang, C. Highly efficient hydrodeoxygenation of lignin-derivatives over Ni-based catalyst. Appl. Catal. B Environ. 2021, 293, 120243. [Google Scholar] [CrossRef]
- Mukherjee, D.; Singuru, R.; Venkataswamy, P.; Damma, D.; Reddy, B.M. Ceria promoted Cu-Ni/SiO2 catalyst for selective hydrodeoxygenation of vanillin. ACS Omega 2019, 4, 4770–4778. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ochoa-Hernández, C.; Víctor, A.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Effect of metal–support interaction on the selective hydrodeoxygenation of anisole to aromatics over Ni-based catalysts. Appl. Catal. B Environ. 2014, 145, 91–100. [Google Scholar] [CrossRef]
- Saidi, M.; Moradi, P. Catalytic hydrotreatment of lignin-derived pyrolysis bio-oils using Cu/γ-Al2O3 catalyst: Reaction network development and kinetic study of anisole upgrading. Int. J. Energy Res. 2021, 45, 8267–8284. [Google Scholar] [CrossRef]
- Ranga, C.; Alexiadis, V.I.; Lauwaert, J.; Lødeng, R.; Thybaut, J.W. Effect of Co incorporation and support selection on deoxygenation selectivity and stability of (Co) Mo catalysts in anisole HDO. Appl. Catal. A Gen. 2019, 571, 61–70. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydin, G. Fossil fuel use and related carbon dioxide emissions: A global perspective. In Proceedings of the 23rd International Mining Congress and Exhibition of Turkey, Antalya, Turkey, 16–19 April 2013; pp. 2137–2141. [Google Scholar]
- Bhadmus, O. Sustainable approaches for surface Transportation mortality in Nigeria. AFRREV STECH Int. J. Sci. Technol. 2013, 2, 113–124. [Google Scholar]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Zhang, P. Adsorption and Desorption Isotherms; KE Group: Bangkok, Thailand, 2016. [Google Scholar]
- Zhang, F.-S.; Nriagu, J.O.; Itoh, H. Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res. 2005, 39, 389–395. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Luo, Y.; Street, J.; Steele, P.; Entsminger, E.; Guda, V. Activated carbon derived from pyrolyzed pinewood char using elevated temperature, KOH, H3PO4, and H2O2. BioResources 2016, 11, 10433–10447. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue–derived biochar. Water Air Soil Pollut. 2021, 232, 236. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Zhang, L.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Hu, G.; Hu, X. Co-presence of hydrophilic and hydrophobic sites in Ni/biochar catalyst for enhancing the hydrogenation activity. Fuel 2021, 293, 120426. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar production and applications in soil fertility and carbon sequestration–a sustainable solution to crop-residue burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Aftab, Z.-e.-H.; Aslam, W.; Aftab, A.; Shah, A.N.; Akhter, A.; Fakhar, U.; Siddiqui, I.; Ahmed, W.; Majid, F.; Wróbel, J. Incorporation of engineered nanoparticles of biochar and fly ash against bacterial leaf spot of pepper. Sci. Rep. 2022, 12, 8561. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Pei, L.; Ying, D.; Xu, X.; Zhao, L.; Jia, J.; Cao, X. Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents. Sci. Rep. 2017, 7, 41523. [Google Scholar] [CrossRef] [PubMed]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. BioResources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Zhang, Y. Decontamination of Cr (VI) facilitated formation of persistent free radicals on rice husk derived biochar. Front. Environ. Sci. Eng. 2019, 13, 22. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar characterization of invasive Pennisetum purpureum grass: Effect of pyrolysis temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- Sanchis, R.; García, T.; Dejoz, A.M.; Vázquez, I.; Llopis, F.J.; Solsona, B. Easy method for the transformation of levulinic acid into gamma-valerolactone using a nickel catalyst derived from nanocasted nickel oxide. Materials 2019, 12, 2918. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Li, X. Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid. Processes 2022, 10, 1542. [Google Scholar] [CrossRef]
- Cheng, F.; Dupont, V.; Twigg, M.V. Temperature-programmed reduction of nickel steam reforming catalyst with glucose. Appl. Catal. A Gen. 2016, 527, 1–8. [Google Scholar] [CrossRef]
- Liu, N.; Song, X.; Wang, C.; Li, K.; Ning, P.; Sun, X.; Wang, F.; Ma, Y. Surface characterization study of corn-straw biochar catalysts for the simultaneous removal of HCN, COS, and CS 2. New J. Chem. 2020, 44, 13565–13575. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abdelfatah, A.M.; Hosny, M.; Fawzy, M. Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega 2022, 7, 8046–8059. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tan, H.; Wang, J.; Yu, S.; Zhou, K. Hydrodeoxygenation of vanillin as a bio-oil model over carbonaceous microspheres-supported Pd catalysts in the aqueous phase and Pickering emulsions. Green Chem. 2014, 16, 2636–2643. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Y.; Fu, Y.; Zhong, Y.; Zhu, W.; Ibrahim, A.A.; El-Shall, M.S. Palladium nanoparticles incorporated within sulfonic acid-functionalized MIL-101 (Cr) for efficient catalytic conversion of vanillin. J. Mater. Chem. A 2015, 3, 17008–17015. [Google Scholar] [CrossRef]
- Vázquez-Fuentes, L.F.; Cortés-Jacome, M.; López-Salinas, E.; Valente, J.S.; Gil, P.M.; Hernández-Cortez, J.; Toledo-Antonio, J.A. Selective vanillin hydrodeoxygenation on synthetic Takovite derived NiAlOx mixed oxide. Top. Catal. 2020, 63, 428–436. [Google Scholar] [CrossRef]
- Kayalvizhi, J.; Pandurangan, A. Hydrodeoxygenation of vanillin using palladium on mesoporous KIT-6 in vapour phase reactor. Mol. Catal. 2017, 436, 67–77. [Google Scholar] [CrossRef]
- Santos, J.; Alda-Onggar, M.; Fedorov, V.; Peurla, M.; Eränen, K.; Mäki-Arvela, P.; Centeno, M.Á.; Murzin, D.Y. Hydrodeoxygenation of vanillin over carbon supported metal catalysts. Appl. Catal. A Gen. 2018, 561, 137–149. [Google Scholar] [CrossRef]
- Aliu, E.; Hart, A.; Wood, J. Kinetics of vanillin hydrodeoxygenation reaction in an organic solvent using a Pd/C catalyst. Ind. Eng. Chem. Res. 2019, 58, 15162–15172. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Y.; Cheng, Y.; Song, W.; Wang, X.; Wang, Z.; Qiu, J. Hydrodeoxygenation of vanillin over carbon nanotube-supported Ru catalysts assembled at the interfaces of emulsion droplets. Catal. Commun. 2014, 47, 28–31. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.; Shyam, S.; Mayuri, N.; Sivaramakrishnan, R.; Pugazhendhi, A. Upgradation of Nostoc punctriforme under subcritical conditions into liquid hydrocarbons (bio-oil) via hydro-deoxygenation: Optimization and engine tests. J. Environ. Chem. Eng. 2021, 9, 105230. [Google Scholar] [CrossRef]
- Varala, S.; Dharanija, B.; Satyavathi, B.; Rao, V.B.; Parthasarathy, R. New biosorbent based on deoiled karanja seed cake in biosorption studies of Zr (IV): Optimization using Box–Behnken method in response surface methodology with desirability approach. Chem. Eng. J. 2016, 302, 786–800. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Pham, V.V.; Do, H.T. Preparation of Ni/biochar catalyst for hydrotreating of bio-oil from microalgae biomass. Catal. Lett. 2016, 146, 2381–2391. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Bocchini, S.; Rizzo, A.M.; Chiaramonti, D.; Pirone, R.; Bensaid, S. Aqueous phase reforming of the residual waters derived from lignin-rich hydrothermal liquefaction: Investigation of representative organic compounds and actual biorefinery streams. Catal. Today 2020, 345, 237–250. [Google Scholar] [CrossRef]














| Catalyst | Surface Area (m2g−1) | Pore Volume (cm3g−1) | Ni Content (%) |
|---|---|---|---|
| Ni/biochar | 74.837 | 0.095 | 15.1 |
| Ni/biochar (KOH) | 123.304 | 0.15 | 15.3 |
| Ni/biochar (H2SO4) | 353.503 | 0.339 | 14.7 |
| Source | DF | Adj ss | Adj MS | F-Value | P-Value Significance |
|---|---|---|---|---|---|
| Model Linear | 9 3 | 106,44.7 9927.7 | 1182.74 3309.23 | 355.07 993.46 | 0.000 S 0.000 S |
| Temperature (°C) | 1 | 9058.6 | 9058.58 | 2719.47 | 0.000 S |
| Pressure (Bar) | 1 | 791.2 | 791.22 | 237.53 | 0.000 S |
| Catalyst Loading (g) | 1 | 77.9 | 77.88 | 23.38 | 0.005 S |
| Square | 3 | 676.1 | 225.36 | 67.65 | 0.000 S |
| Temp. (°C) × Temp. (°C) | 1 | 595.6 | 595.61 | 178.81 | 0.000 S |
| Pressure (Bar) × Pressure (Bar) | 1 | 48.1 | 48.10 | 14.44 | 0.013 S |
| Cat. Loading (g) × Cat. Loading (g) | 1 | 7.7 | 7.67 | 2.30 | 0.190 NS |
| 2-Way Interaction | 3 | 40.9 | 13.64 | 4.09 | 0.082 NS |
| Temp. (°C) × Pressure (Bar) | 1 | 28.6 | 28.62 | 8.59 | 0.033 S |
| Temp. (°C) × Catalyst Loading (g) | 1 | 4.6 | 4.62 | 1.39 | 0.292 NS |
| Pressure (Bar) × Cat.Loading (g) | 1 | 7.7 | 7.67 | 2.30 | 0.190 NS |
| Error | 5 | 16.7 | 3.33 |
| S | R2 | R2 (adj.) | R2 (pred.) |
|---|---|---|---|
| 2.14 | 99.66 | 99.40 | 98.65 |
| S/no. | Temperature (°C) | Pressure (bar) | Catalyst (g) | Calculated Value | Experimental Value |
|---|---|---|---|---|---|
| 1 | 120 | 40 | 0.4 | 34.04 | 44.01 |
| 2 | 135 | 30 | 0.6 | 45.14 | 54.48 |
| 3 | 140 | 50 | 0.8 | 79 | 84.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudi, I.; Hart, A.; Ingram, A.; Wood, J. Catalytic Hydrodeoxygenation of Vanillin, a Bio-Oil Model Compound over Renewable Ni/Biochar Catalyst. Catalysts 2023, 13, 171. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010171
Mudi I, Hart A, Ingram A, Wood J. Catalytic Hydrodeoxygenation of Vanillin, a Bio-Oil Model Compound over Renewable Ni/Biochar Catalyst. Catalysts. 2023; 13(1):171. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010171
Chicago/Turabian StyleMudi, Ismaila, Abarasi Hart, Andrew Ingram, and Joseph Wood. 2023. "Catalytic Hydrodeoxygenation of Vanillin, a Bio-Oil Model Compound over Renewable Ni/Biochar Catalyst" Catalysts 13, no. 1: 171. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010171