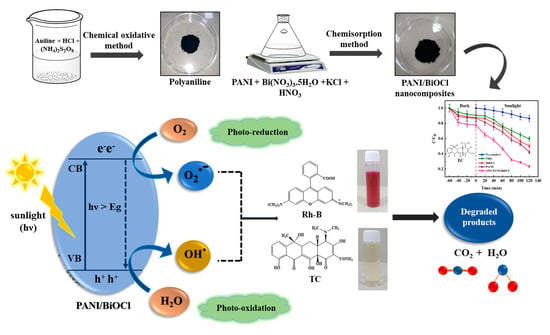
Solar Light-Induced Photocatalytic Response of BiOCl/PANI Composite towards the Degradation of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
2.1. XPS Analysis
2.2. XRD Analysis
2.3. Photoluminescence Spectroscopy
2.4. UV–Visible Diffuse Reflectance Spectroscopy
2.5. Nitrogen Adsorption/Desorption Analysis
2.6. FESEM and TEM Analysis
2.7. EDX and Elemental Mapping
2.8. Photocatalytic Study
2.9. Effect of pH
2.10. Effect of Catalyst Concentration
2.11. Plausible Mechanism
2.12. Reusability Studies
3. Experimental Section
3.1. Materials and Chemicals
3.2. Synthesis of Polyaniline
3.3. Synthesis of PANI/BiOCl Composites
3.4. Characterization Methods
3.5. Photocatalytic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the Environment: Occurrence, Perils, and Eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Sharma, S.; Shetti, N.P.; Basu, S.; Nadagouda, M.N.; Aminabhavi, T.M. Remediation of per- and Polyfluoroalkyls (PFAS) via Electrochemical Methods. Chem. Eng. J. 2022, 430, 132895. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-Energy Nexus for Circular Economy and Environmental Protection: Recent Trends in Hydrogen Energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S. Visible-Light-Induced Photocatalytic Response of Easily Recoverable Mn2O3/SiO2 Monolith in Centimeter-Scale towards Degradation of Ofloxacin: Performance Evaluation and Product Analysis. Chemosphere 2022, 307, 135973. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Fabrication of Centimeter-Sized Sb2S3/SiO2 Monolithic Mimosa Pudica Nanoflowers for Remediation of Hazardous Pollutants from Industrial Wastewater. J. Clean. Prod. 2021, 280, 124525. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, Y.; Liu, W.; Pan, F.; Sun, P.; Fu, J. An Overview of Nanomaterials Applied for Removing Dyes from Wastewater. Environ. Sci. Pollut. Res. 2017, 24, 15882–15904. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, B. Photocatalytic Degradation of Dyes: An Overview. Curr. Catal. 2018, 7, 99–121. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Visible-Light-Driven Efficient Photocatalytic Abatement of Recalcitrant Pollutants by Centimeter-Length MoO3/SiO2 Monoliths with Long Service Life. Appl. Mater. Today 2021, 23, 101033. [Google Scholar] [CrossRef]
- Saadati, F.; Keramati, N.; Ghazi, M.M. Influence of Parameters on the Photocatalytic Degradation of Tetracycline in Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 757–782. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Singla, S.; Singh, P.; Basu, S.; Devi, P. BiVO4/MoSe2 Photocatalyst for the Photocatalytic Abatement of Tetracycline and Photoelectrocatalytic Water Splitting. Mater. Chem. Phys. 2023, 295, 127111. [Google Scholar] [CrossRef]
- Singla, S.; Basu, S.; Devi, P. Solar Light Responsive 2D/2D BiVO4/SnS2 Nanocomposite for Photocatalytic Elimination of Recalcitrant Antibiotics and Photoelectrocatalytic Water Splitting with High Performance. J. Ind. Eng. Chem. 2022, 41, 101284. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Z.; Duan, J.; He, X. Adsorption and Photocatalytic Degradation Mechanism of Magnetic Graphene oxide/ZnO Nanocomposites for Tetracycline Contaminants. Chem. Eng. J. 2020, 400, 125952. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ramezani Motlagh, H.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced Photocatalytic Degradation of Tetracycline and Real Pharmaceutical Wastewater Using MWCNT/TiO2 Nano-Composite. J. Environ. Manage. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S. MoS2/WO3 Heterojunction with the Intensified Photocatalytic Performance for Decomposition of Organic Pollutants under the Broad Array of Solar Light. J. Clean. Prod. 2021, 324, 129290. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ahamed, S.T.; Mitra, M.; Kulsi, C.; Mondal, A.; Banerjee, D. Visible-Light Influenced Photocatalytic Activity of Polyaniline -Bismuth Selenide Composites for the Degradation of Methyl Orange, Rhodamine B and Malachite Green Dyes. Appl. Surf. Sci. 2019, 470, 472–483. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Construction of an Efficient and Durable Hierarchical Porous CuO/SiO2 Monolith for Synergistically Boosting the Visible-Light-Driven Degradation of Organic Pollutants. Sep. Purif. Technol. 2021, 279, 119759. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, S.; Rathi, A.; Saha, P.; Basu, S. Solar-Light-Driven Photocatalysis by Sb2S3/carbon Based Composites towards Degradation of Noxious Organic Pollutants. Mater. Chem. Phys. 2021, 273, 125120. [Google Scholar] [CrossRef]
- Monga, D.; Sharma, S.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Advances in Transition Metal Dichalcogenide-Based Two-Dimensional Nanomaterials. Mater. Today Chem. 2021, 19, 100399. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly Reusable Visible Light Active Hierarchical Porous WO3/SiO2 Monolith in Centimeter Length Scale for Enhanced Photocatalytic Degradation of Toxic Pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Xin, X.; Wang, L.; Li, H.; Zheng, X.; Jiang, Y. Immobilization Laccase on Heterophase TiO2 Microsphere as a Photo-Enzyme Integrated Catalyst for Emerging Contaminants Degradation under Visible Light. Appl. Mater. Today 2020, 21, 100810. [Google Scholar] [CrossRef]
- Kundu, A.; Sharma, S.; Basu, S. Modulated BiOCl Nanoplates with Porous G-C3N4 Nanosheets for Photocatalytic Degradation of Color/colorless Pollutants in Natural Sunlight. J. Phys. Chem. Solids 2021, 154, 110064. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent Developments in Heterogeneous Photocatalytic Water Treatment Using Visible Light-Responsive Photocatalysts: A Review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Aanchal; Basu, S. Synthesis of Fe2O3/TiO2 Monoliths for the Enhanced Degradation of Industrial Dye and Pesticide via Photo-Fenton Catalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 32–42. [Google Scholar] [CrossRef]
- Zarezadeh, S.; Habibi-Yangjeh, A.; Mousavi, M. BiOBr and AgBr Co-Modified ZnO Photocatalyst: A Novel Nanocomposite with P-N-N Heterojunctions for Highly Effective Photocatalytic Removal of Organic Contaminants. J. Photochem. Photobiol. A Chem. 2019, 379, 11–23. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Zhang, D.; Su, C.; Yao, S.; Li, H.; Pu, X.; Geng, Y. Facile in Situ Chemical Transformation Synthesis, Boosted Charge Separation, and Increased Photocatalytic Activity of BiPO4/BiOCl P-N Heterojunction Photocatalysts under Simulated Sunlight Irradiation. J. Phys. Chem. Solids 2020, 147, 109630. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Zhang, Y.; Gu, Y.; An, Y. BiOCl-Based Photocatalysts: Synthesis Methods, Structure, Property, Application, and Perspective. Inorg. Chem. Commun. 2022, 138, 109277. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Single-Crystalline 2D BiOCl Nanorods Decorated with 2D MoS2 Nanosheets for Visible Light-Driven Photocatalytic Detoxification of Organic and Inorganic Pollutants. FlatChem 2021, 28, 100267. [Google Scholar] [CrossRef]
- Lee, S.; Chang, C.-J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers 2019, 11, 206. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarrintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive Polymers in Water Treatment: A Review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Park, Y.; Numan, A.; Ponomarev, N.; Iqbal, J.; Khalid, M. Enhanced Photocatalytic Performance of PANI-rGO-MnO2 Ternary Composite for Degradation of Organic Contaminants under Visible Light. J. Environ. Chem. Eng. 2021, 9, 106006. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H.; Sharma, R.; Kumari, R. Influence of Polyaniline on the Photocatalytic Properties of Metal Nanocomposites: A Review. Colloid Interface Sci. Commun. 2021, 40, 100339. [Google Scholar] [CrossRef]
- Tang, M.; Li, X.; Deng, F.; Han, L.; Xie, Y.; Huang, J.; Chen, Z.; Feng, Z.; Zhou, Y. BiPO4/Ov-BiOBr High-Low Junctions for Efficient Visible Light Photocatalytic Performance for Tetracycline Degradation and H2O2 Production. Catalysts 2023, 13, 634. [Google Scholar] [CrossRef]
- Xie, L.; Wu, P.; Lei, Q.; Xu, C.; Huang, W.; Chen, X.; Yang, K.; He, H. Constructing Z-Scheme 0D/2D TiO2 Nanoparticles/Bi2O3 Nanosheet Heterojunctions with Enhanced Visible Light Induced Photocatalytic Antibiotics Degradation and Hydrogen Evolution. Catalysts 2023, 13, 583. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Yilmaz, M.; Ramírez-Coronel, A.A.; Al-Awsi, G.R.L.; Alwaily, E.R.; Asghari, A.; Balarak, D. Degradation of Amoxicillin under a UV or Visible Light Photocatalytic Treatment Process Using Fe2O3/bentonite/TiO2: Performance, Kinetic, Degradation Pathway, Energy Consumption, and Toxicology Studies. Optik 2023, 272, 170230. [Google Scholar] [CrossRef]
- Chen, R.; Ding, S.; Fu, N.; Ren, X. Preparation of a G-C3N4/Ag3PO4 Composite Z-Type Photocatalyst and Photocatalytic Degradation of Ofloxacin: Degradation Performance, Reaction Mechanism, Degradation Pathway and Toxicity Evaluation. J. Environ. Chem. Eng. 2023, 11, 109440. [Google Scholar] [CrossRef]
- Tanwar, R.; Kumar, S.; Mandal, U.K. Photocatalytic Activity of PANI/Fe 0 Doped BiOCl under Visible Light-Degradation of Congo Red Dye. J. Photochem. Photobiol. A Chem. 2017, 333, 105–116. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of Methyl Orange with PANI-Modified BiOCl Photocatalyst under Visible Light Irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Wang, J.; Hao, X.; Jiang, Y.; Zhang, D.; Ren, L.; Gong, J.; Wu, X.; Zhang, Y.; Tong, Z. Synthesis, Structure, and Photocatalytic Activity of PANI/BiOCl Nanocomposites. Mater. Res. Express 2019, 6, 0850c1. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Q.; Li, B.; Dong, L.; Fan, M.; Zhang, F. Synthesis and Characterization of Cu2O–modified Bi2O3 Nanospheres with Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2015, 357, 1072–1079. [Google Scholar] [CrossRef]
- Cui, J.; Tao, S.; Yang, X.; Yu, X.; Sun, S.; Yang, Q.; Wei, W.; Liang, S. Facile Construction of Nickel-Doped Hierarchical BiOCl Architectures for Enhanced Visible-Light-Driven Photocatalytic Activities. Mater. Res. Bull. 2021, 138, 111208. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Chi, M.; Yang, Z.; Nie, G.; Lu, X.; Wang, C. Fabrication of Au Nanoparticles Supported on CoFe2O4 Nanotubes by Polyaniline Assisted Self-Assembly Strategy and Their Magnetically Recoverable Catalytic Properties. Appl. Surf. Sci. 2016, 363, 578–585. [Google Scholar] [CrossRef]
- Tanwar, R.; Mandal, U.K. Photocatalytic Activity of Ni0.5Zn0.5Fe2O4 @polyaniline Decorated BiOCl for Azo Dye Degradation under Visible Light—Integrated Role and Degradation Kinetics Interpretation. RSC Adv. 2019, 9, 8977–8993. [Google Scholar] [CrossRef] [PubMed]
- Namdarian, A.; Goljanian Tabrizi, A.; Arsalani, N.; Khataee, A.; Mohammadi, A. Synthesis of PANi Nanoarrays Anchored on 2D BiOCl Nanoplates for Photodegradation of Congo Red in Visible Light Region. J. Ind. Eng. Chem. 2020, 81, 228–236. [Google Scholar] [CrossRef]
- Yaghoubi-berijani, M.; Bahramian, B. Preparation and Measurement of Properties of BiOBr/BiOCl/PANI Ternary Nanocomposite for Highly Efficient Visible Light Photocatalytic Applications. Res. Chem. Intermed. 2021, 47, 2311–2330. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Combination of MoS2 Nanopetals with Ag Nanoparticles Decorated Graphene Oxide for Boosting Photocatalytic Abatement of Recalcitrant Pollutants under Visible Light Irradiation. Adv. Powder Technol. 2022, 33, 103555. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and Stable Photocatalytic Degradation of Tetracycline Wastewater by 3D Polyaniline/Perylene Diimide Organic Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Ji, X.; Huang, S.; Xia, J.; Xie, M.; Yan, J.; Xu, H.; Li, H. Conjugated Conducting Polymers PANI Decorated Bi12O17Cl2 Photocatalyst with Extended Light Response Range and Enhanced Photoactivity. Appl. Surf. Sci. 2019, 464, 552–561. [Google Scholar] [CrossRef]
- Bouziani, A.; Yahya, M.; Bianchi, C.L.; Falletta, E.; Celik, G. Ternary Polyaniline@Bi2O3-BiOCl Nanocomposites as Innovative Highly Active Photocatalysts for the Removal of the Dye under Solar Light Irradiation. Nanomaterials 2023, 13, 713. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic Fabrication of ZnO and Gold Decorated ZnO Nanoparticles for Photocatalytic Degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Bui, D.P.; Huu Pham, H.; Minh Cao, T.; Van Pham, V. Preparation of Conjugated Polyvinyl chloride/TiO2 Nanotubes for Rhodamine B Photocatalytic Degradation under Visible Light. J. Chem. Technol. Biotechnol. 2020, 95, 2707–2714. [Google Scholar] [CrossRef]
- Hu, L.; Chen, F.; Hu, P.; Zou, L.; Hu, X. Hydrothermal Synthesis of SnO2/ZnS Nanocomposite as a Photocatalyst for Degradation of Rhodamine B under Simulated and Natural Sunlight. J. Mol. Catal. A Chem. 2016, 411, 203–213. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Yin, B.; Li, D.; Zhang, Z.; Mao, B.; Fan, W.; Gu, W.; Shi, W. Promoting Visible-Light-Induced Photocatalytic Degradation of Tetracycline by an Efficient and Stable Beta-Bi2O3@g-C3N4 Core/shell Nanocomposite. Chem. Eng. J. 2018, 338, 137–146. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, C.; Yang, J.; Mo, Q.; Zhang, Y.; Tang, Y. Visible Light Responsive Bi2WO6/BiOCl Heterojunction with Enhanced Photocatalytic Activity for Degradation of Tetracycline and Rohdamine B. Inorg. Chem. Commun. 2018, 93, 136–139. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Pandey, G. Synthesis, Characterization of Titania/Polyaniline/GO Nanocomposites, and Its Photocatalytic Activity under UV-Visible Light. Macromol. Symp. 2018, 379, 1600192. [Google Scholar] [CrossRef]









| Sample | Surface Area (m2/g) | Mean Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| PANI | 17 | 38.80 | 0.174 |
| BiOCl | 10 | 13.56 | 0.086 |
| 15% PANI/BiOCl | 25 | 42.54 | 0.263 |
| Photocatalyst | Pollutant Degraded (conc.) | Catalyst Concentration (g/L) | Light Source | Time (min) | Degradation Efficiency (%) | Rate Constant (min−1) | Reference |
|---|---|---|---|---|---|---|---|
| Au-ZnO | Rh-B (10 ppm) | 0.3 | Visible | 180 | 78.12 | 0.0246 | [51] |
| Conjugated polyvinyl chloride/TiO2 nanotubes | Rh-B (20 ppb) | 0.3 | Visible | 180 | 90 | 0.015 | [52] |
| SnO2/ZnS | Rh-B (5 ppm) | 2 | Sunlight | 100 | 90 | 0.046 | [53] |
| β-Bi2O3/g-C3N4 | TC (10 ppm) | 0.5 | Visible | N/A | 80 | 0.0311 | [54] |
| Multi wall carbon nanotubes/TiO2 | TC (10 ppm) | 0.2 | Visible | 100 | 74 | 0.064 | [14] |
| Bi2WO6/BiOCl | TC (50 ppm) | N/A | Visible | 90 | 61.5 | N/A | [55] |
| BiOCl/PANI | Rh-B (5 ppm) | 0.4 | Sunlight | 90 | 97 | 0.0236 | Present work |
| BiOCl/PANI | TC (10 ppm) | 0.5 | Sunlight | 90 | 77 | 0.0106 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, J.; Sharma, S.; Basu, S. Solar Light-Induced Photocatalytic Response of BiOCl/PANI Composite towards the Degradation of Tetracycline. Catalysts 2023, 13, 795. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050795
Goyal J, Sharma S, Basu S. Solar Light-Induced Photocatalytic Response of BiOCl/PANI Composite towards the Degradation of Tetracycline. Catalysts. 2023; 13(5):795. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050795
Chicago/Turabian StyleGoyal, Janis, Surbhi Sharma, and Soumen Basu. 2023. "Solar Light-Induced Photocatalytic Response of BiOCl/PANI Composite towards the Degradation of Tetracycline" Catalysts 13, no. 5: 795. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050795