Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Photocatalysts
2.1.1. XRD Analysis
2.1.2. Morphology—Surface Analysis of the Photocatalysts
2.1.3. FT-IR Spectroscopy
2.1.4. UV-Vis Spectra
2.1.5. Determination of •OH by Fluorescence Measurements
2.2. Photocatalytic Activity
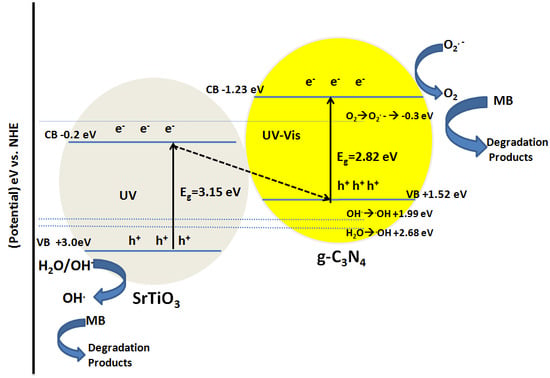
2.3. Mechanism Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of g-C3N4 and g-C3N4/SrTiO3 Heterojunctions
3.3. Texture Characterization of the Heterojunctions
3.4. Fourier Transform. Infrared Spectroscopy (FT-IR)
3.5. UV-Vis.-Diffuse Reflectance Measurements
3.6. Photocatalytic Experiments and Analytical Methods
3.7. Determination of •OH Radicals by Fluorescence Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis —Past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Mohammad, N.; Arami, M. Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. J. Photochem. Photobiol. B Biol. 2009, 94, 20–24. [Google Scholar]
- Molinari, R.; Borgese, M.; Drioli, E.; Palmisano, L.; Schiavello, M. Hybrid processes coupling photocatalysis and membranes for degradation of organic pollutants in water. Catal. Today 2002, 75, 77–85. [Google Scholar]
- Kato, H.; Sasaki, Y.; Shirakura, N.; Kudo, A. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting. J. Mater. Chem. A 2013, 1, 12327–12333. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, S.; Wang, D.; Wang, X.; Shi, J.; Zhang, F.; Han, H.; Li, C. Composite Sr2TiO4/SrTiO3 (La, Cr) heterojunction based photocatalyst for hydrogen production under visible light irradiation. J. Mater. Chem. A 2013, 1, 7905–7912. [Google Scholar] [CrossRef]
- Maeda, K. Rhodium-Doped Barium Titanate Perovskite as a Stable p-Type Semiconductor Photocatalyst for Hydrogen Evolution under Visible Light. ACS Appl. Mater. Interfaces 2014, 6, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Hamm, I.; Wark, M.; Mudring, A.-V. Low-temperature route to metal titanate perovskite nanoparticles for photocatalytic applications. Appl. Catal. B Environ. 2015, 178, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Sulaeman, U.; Yin, S.; Sato, T. Solvothermal synthesis and photocatalytic properties of chromium-doped SrTiO3 nanoparticles. Appl. Catal. B Environ. 2011, 105, 206–210. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Yan, S.; Yu, T.; Zou, Z. Elements doping to expand the light response of SrTiO3. J. Photochem. Photobiol. A: Chem. 2014, 275, 65–71. [Google Scholar] [CrossRef]
- Wang, J.; Yin, S.; Komatsu, M.; Sato, T. Lanthanum and Nitrogen Co-Doped SrTiO3 Powders as Visible Light Sensitive Photocatalyst. J. Eur. Ceram. Soc. 2005, 25, 3207–3212. [Google Scholar] [CrossRef]
- Cao, T.; Li, Y.; Wang, C.; Shao, C.; Liu, Y. A facile in situ hydrothermal method to SrTiO3/TiO2 nanofiber heterostructures with high photocatalytic activity. Langmuir 2011, 27, 2946–2952. [Google Scholar] [CrossRef] [PubMed]
- Van Benthem, K.; Elsässer, C.; French, R. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. A Chem. 2002, 148, 71–77. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Philos. Trans. R. Soc. Lond. A 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Puangpetch, T.; Chavadej, S.; Sreethawong, T. Hydrogen production over Au-loaded mesoporous-assembled SrTiO3 nanocrystal photocatalyst: Effects of molecular structure and chemical properties of hole scavengers. Energy Convers. Manag. 2011, 52, 2256–2261. [Google Scholar] [CrossRef]
- Kappadan, S.; Gebreab, T.W.; Thomas, S.; Kalarikkal, N. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants. Mat. Sci. Semicond. Proc. 2016, 51, 42–47. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Liu, J.; Wang, T.; Qu, W.; Li, Z. DFT study on electronic structures and optical absorption properties of C, S cation- doped SrTiO3. Cent. Eur. J. Phys. 2009, 7, 762–767. [Google Scholar] [CrossRef]
- Zhang, J.; Bang, J.H.; Tang, C.; Kamat Pr., V. Tailored TiO2-SrTiO3 Heterostructure Nanotube Arrays for Improved Photoelectrochemical Performance. ACS Nano 2010, 4, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Boumaza, S.; Boudjemaa, A.; Bouguelia, A.; Bouarab, R.; Trari, M. Visible light induced hydrogen evolution on new hetero-system ZnFe2O4/SrTiO3. Appl. Energy 2010, 87, 2230–2236. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2014, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride(g-C3N4)-Based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Lin, L.; Zheng, Y.; Yang, P.; Fang, Y.; Wang, X. Tri-s-triazine-based crystalline carbon nitride nanosheets for an improved hydrogen evolution. Adv. Mater. 2017, 29, 1700008. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ou, H.; Fang, Y.; Wang, X. A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Peng, X.; Zeng, G.; Zhong, H.; Liang, J.; Ren, M.M. Graphene-based materials: Fabrication, characterization and application for the decontamination of wastewater and waste gas and hydrogen storage/generation. Adv. Colloid Interface Sci. 2013, 195–196, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, J.; Zeng, H. Two-dimensional semiconductors: Recent progress and future perspectives. J. Mater. Chem. C 2013, 1, 2952–2969. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nano composites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; Zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal-organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 25638. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Gong, P.; Sun, J.; Wang, J.; Yang, S. Design and synthesis of Ni-MOF/CNT composites and rGO/carbon nitride composites for an asymmetric supercapacitor with high energy and power density. J. Mater. Chem. A: Chem. 2015, 3, 13874–13883. [Google Scholar] [CrossRef]
- Dyjak, S.; Kicinski, W.; Huczko, A. Thermite-driven melamine condensation to CxNyHz graphitic ternary polymers: Towards an instant, large-scale synthesis of g-C3N4. J. Mater. Chem. A. 2015, 3, 9621–9631. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Tian, K.; Hu, J.-Y.; Jiang, H. Significant enhancement of photoreactivity of graphitic carbon nitride catalysts under acidic conditions and the underlying H+-mediated mechanism. Chemosphere 2015, 141, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.-K. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 2015, 7, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Yao, W.; Zhu, Y. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite. Appl. Catal. B Environ. 2015, 166–167, 366–373. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-C.; Li, Z.-S.; Zou, Z.-G. Photodegradation of Rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Mori, T.; Ye, J.-H.; Antonietti, M. Phosphorus-doped carbon nitride solid: Enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Li, Q.-Y.; Iwai, H.; Kako, T.; Ye, J.-H. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light. Sci. Technol. Adv. Mater. 2011, 12, 034401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide photocatalysts active in visible light. Angew. Chem. Int. Ed. Engl. 2008, 47, 9975–9978. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Yue, B.; Iwai, H.; Kako, T.; Ye, J.H. Carbon nitride polymers sensitized with N-doped tantalic acid for visible light- Induced photocatalytic hydrogen evolution. J. Phys. Chem. C 2010, 114, 4100–4105. [Google Scholar] [CrossRef]
- Rakibuddin, Md.; Kim, H.; Khan, M.E. Graphite-like carbon nitride (C3N4) modified N-doped LaTiO3 nanocomposite for higher visible light photocatalytic and photo-electrochemical performance. Appl. Surf. Sci. 2018, 452, 400–412. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Liu, Y.; Tan, Y.Z.; Yuan, X.; Chew, J.W. Quasi-polymeric construction of stable perovskite-type LaFeO3/g-C3N4 heterostructured photocatalyst for improved Z-scheme photocatalytic activity via solid p-n heterojunction interfacial effect. J. Hazard. Mater. 2018, 347, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-structured CaTiO3 coupled with g-C3N4 as a heterojunction photocatalyst for organic pollutant degradation. Beilstein J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tonda, S.; Baruah, A.; Kumar, B.; Shanker, V. Synthesis of novel and stable g-C3N4/N-doped SrTiO3 hybrid nanocomposites with improved photocurrent and photocatalytic activity under visible light irradiation. Dalton Trans. 2014, 43, 16105–16114. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, X.; Ning, X.; Zhan, L.; Chen, J.; Li, Z. Constructing a direct Z-scheme La2NiO4/g-C3N4 hybrid photocatalyst with boosted visible light photocatalytic activity. Sep. Purif. Technol. 2018, 201, 327–335. [Google Scholar] [CrossRef]
- Chen, X.; Tan, P.; Zhou, B.; Dong, H.; Pan, J.; Xiong, X. A green and facile strategy for preparation of novel and stable Cr-doped SrTiO3/g-C3N4 hybrid nanocomposites with enhanced visible light photocatalytic activity. J. Alloys Compd. 2015, 647, 456–462. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Randorn, C.; Irvine, J.T.S. g-C3N4 coated SrTiO3 as an efficient photocatalyst for H2 production in aqueous solution under visible light irradiation. Int. J. Hydrog. Energy 2011, 36, 13501–13507. [Google Scholar] [CrossRef]
- Kang, H.W.; Lim, S.N.; Song, D.; Park, S.B. Organic-inorganic composite of g-C3N4-SrTiO3: Rh photocatalyst for improved H2 evolution under visible light irradiation. Int. J. Hydrog. Energy 2012, 37, 11602–11610. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef] [Green Version]
- Mittemeijer, E.J.; Welzel, U. The “state of the art” of the diffraction analysis of crystallite size and lattice strain. Z. Kristallogr. 2008, 223, 552–560. [Google Scholar] [CrossRef]
- Hall, W.H. X-ray line broadening in metals. Proc. Philos. Soc. Lond. 1949, 62, 741–743. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Acharya, S.; Mansingh, S.; Parida, K.M. The enhanced photocatalytic activity of g-C3N4-LaFeO3 for water reduction reaction through mediator free Z-scheme mechanism. Inorg. Chem. Fron. 2017, 4, 1022–1032. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.J.; Dai, J.F. Enhanced photocatalytic activity of BaTiO3@g-C3N4 for the degradation of methyl orange under simulated sunlight irradiation. J. Alloys Compd. 2015, 622, 1098–1104. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Jia, A.; Liang, X.; Su, Z.; Zhu, T.; Liu, S. Synthesis and the effect of calcination temperature on the physical–chemical properties and photocatalytic activities of Ni, La codoped SrTiO3. J. Hazard. Mater. 2010, 178, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Wang, X.; Zou, Z. Ag@SrTiO3 nanocomposite for super photocatalytic degradation of organic dye and catalytic reduction of 4-nitrophenol. New J. Chem. 2017, 41, 5678–5687. [Google Scholar] [CrossRef]
- Yang, M.; Jin, X.-Q. Improvement of visible light-induced photocatalytic performance by Cr-doped SrTiO3−carbon nitride intercalation compound (CNIC) composite. J. Cent. South Univ. 2016, 23, 310–316. [Google Scholar] [CrossRef]
- Sun, L.; Qi, Y.; Jia, C.J.; Jin, Z.; Fan, W. Enhanced visible-light photocatalytic activity of g-C3N4/Zn2GeO4 heterojunctions with effective interfaces based on band match. Nanoscale 2014, 6, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Yang, Y.; Zhang, Y.; He, D.; An, Q.; Cao, G. Seed-induced growing various TiO2 nanostructures on g-C3N4 nanosheets with much enhanced photocatalytic activity under visible light. J. Hazard. Mater. 2015, 292, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Wu, Y.; Wu, H.; Wu, L.; Tan, P.; Pan, J.; Xiong, X. Facile fabrication of novel porous graphitic carbon nitride/copper sulfide nanocomposites with enhanced visible light driven photocatalytic performance. J. Colloid Interface Sci. 2016, 476, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (PZC) of Metal (Hydr)Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]











| Code Name | Crystal Phase | Space Group | % Phase | a | b | c | Unit Cell Volume (A3) | E % | R % | Crystal Size (nm) | % Strain | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10CNSTO | SrTiO3 | cubic | 100 | 3.9114 | 3.9114 | 3.9114 | 59.84 | 19.16 | 26.87 | 17.1 | −0.074 | −0.359 |
| 20CNSTO | SrTiO3 | cubic | 100 | 3.9104 | 3.9105 | 3.9105 | 59.80 | 16.16 | 23.53 | 25.5 | −0.037 | −0.320 |
| 30CNSTO | SrTiO3 | cubic | 100 | 3.9093 | 3.9093 | 3.9093 | 59.74 | 13.77 | 21.22 | 29.0 | 0.000 | 0.060 |
| 40CNSTO | SrTiO3 | cubic | 100 | 3.9099 | 3.9099 | 3.9099 | 59.77 | 16.00 | 24.99 | 24.9 | −0.005 | −0.106 |
| 50CNSTO | SrTiO3 | cubic | 100 | 3.9103 | 3.9103 | 3.9103 | 59.79 | 16.04 | 25.29 | 24.2 | −0.040 | −0.332 |
| STO | SrTiO3 | cubic | 100 | 3.9087 | 3.9087 | 3.9087 | 59.7 | 14.79 | 20.62 | 27.5 | 0.016 | 0.930 |
| PZC | Eg (eV) | ||
|---|---|---|---|
| Catalysts | g-C3N4 | SrTiO3 | |
| 10CNSTO | 8.02 | 2.80 | 3.40 |
| 20CNSTO | 7.90 | 2.80 | 3.28 |
| 30CNSTO | 7.87 | 2.82 | 3.28 |
| 40CNSTO | 7.79 | 2.84 | 3.20 |
| 50CNSTO | 7.65 | 2.84 | 3.21 |
| CN | 4.63 | 2.82 | |
| STO | 9.33 | 3.15 | |
| UV-Vis | Visible | |||||
|---|---|---|---|---|---|---|
| Catalysts | K (min−1) | t1/2 (min) | R2 | K (min−1) | t1/2 (min) | R2 |
| 10CNSTO | 0.0150 | 46.2 | 0.9804 | 0.0050 | 138.6 | 0.9690 |
| 20CNSTO | 0.0220 | 31.5 | 0.9886 | 0.0071 | 97.6 | 0.9780 |
| 30CNSTO | 0.0181 | 38.3 | 0.9885 | 0.0058 | 119.5 | 0.9893 |
| 40CNSTO | 0.0170 | 40.8 | 0.9932 | 0.0055 | 126.0 | 0.9766 |
| 50CNSTO | 0.0160 | 43.3 | 0.9797 | 0.0049 | 141.4 | 0.9942 |
| STO | 0.0140 | 49.5 | 0.9932 | - | - | - |
| CN | 0.0146 | 47.5 | 0.9996 | 0.0055 | 126.0 | 0.9986 |
| 20CNSTO | ||||
|---|---|---|---|---|
| Scavengers | Radicals Scavenge | k (min−1) | % Δk | R2 |
| No scavenger | - | 0.0220 | 0 | 0.9886 |
| IPA | OH• | 0.0148 | 32.7 | 0.9996 |
| FA | h+ | 0.0086 | 60.9 | 0.9803 |
| N2 | O2•− | 0.0156 | 29.1 | 0.9773 |
| Acetonitrile/N2 | OH•/ O2•− | 0.0050 | 77.3 | 0.9052 |
| SODred | O2•− | 0.0309 | 40.4 | 0.9936 |
| NaN3 | OH• + 1O2 | 0.0130 | 40.9 | 0.9814 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstas, P.-S.; Konstantinou, I.; Petrakis, D.; Albanis, T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts 2018, 8, 554. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8110554
Konstas P-S, Konstantinou I, Petrakis D, Albanis T. Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation. Catalysts. 2018; 8(11):554. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8110554
Chicago/Turabian StyleKonstas, Panagiotis-Spyridon, Ioannis Konstantinou, Dimitrios Petrakis, and Triantafyllos Albanis. 2018. "Synthesis, Characterization of g-C3N4/SrTiO3 Heterojunctions and Photocatalytic Activity for Organic Pollutants Degradation" Catalysts 8, no. 11: 554. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8110554