Tuning Sn-Cu Catalysis for Electrochemical Reduction of CO2 on Partially Reduced Oxides SnOx-CuOx-Modified Cu Electrodes
Abstract
:1. Introduction
2. Results and Discussion
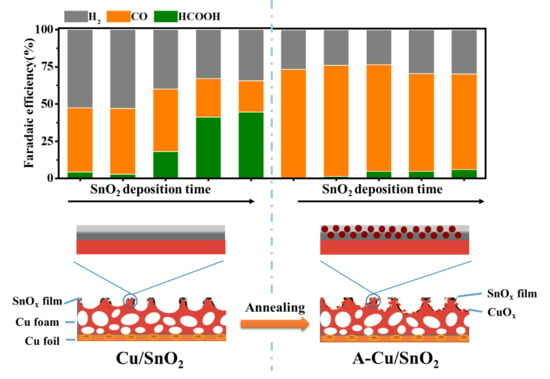
2.1. Fabrication and Characterization of Cu/SnO2 and A-Cu/SnO2 Electrodes
2.2. LSV Analysis for Cu/SnO2 and A-Cu/SnO2
2.3. CO2 Reduction Activity and Product Selectivity on Cu/SnO2 and A-Cu/SnO2
3. Materials and Methods
3.1. Materials
3.2. Electrode Preparation
3.2.1. Fabrication of Cu Foam
3.2.2. Fabrication of Cu/SnO2 and A-Cu/SnO2 Electrodes
3.3. Physical and Chemical Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Pacala, S.; Socolow, R. Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Gong, J. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angew. Chem. Int. Ed. 2017, 129, 11482–11511. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Huang, Y.; Handoko, A.D.; Hirunsit, P.; Yeo, B.S. Electrochemical reduction of CO2 using copper single-crystal surfaces: effects of CO* coverage on the selective formation of ethylene. ACS Catal. 2017, 7, 1749–1756. [Google Scholar] [CrossRef]
- Li, Y.; Cui, F.; Ross, M.B.; Kim, D.; Sun, Y.; Yang, P. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 2017, 17, 1312–1317. [Google Scholar] [CrossRef]
- Handoko, A.D.; Ong, C.W.; Huang, Y.; Lee, Z.G.; Lin, L.; Panetti, G.B.; Yeo, B.S. Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C 2016, 120, 20058–20067. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Chen, C.S.; Handoko, A.D.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and selective electrochemical reduction of carbon dioxide to ethylene on copper mesocrystals. Catal. Sci. Technol. 2015, 5, 161–168. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S. Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef]
- He, J.; Johnson, N.J.J.; Huang, A.; Berlinguette, C.P. Electrocatalytic alloys for CO2 reduction. ChemSusChem 2018, 11, 48–57. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, Y.; Handoko, A.D.; Goh, G.K.L.; Yeo, B.S. Rational design of sulfur-doped copper catalysts for the selective electroreduction of carbon dioxide to formate. ChemSusChem 2018, 11, 320–326. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Y.; Ren, D.; Handoko, A.D.; Seh, Z.W.; Hirunsit, P.; Yeo, B.S. On the role of sulfur for the selective electrochemical reduction of CO2 to formate on CuSx catalysts. ACS Appl. Mater. Interfaces 2018, 10, 28572–28581. [Google Scholar]
- Feaster, J.T.; Shi, C. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Sarfraz, S.; Garcia-Esparza, A.T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu–Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wallace, G.G. Tin nanoparticles decorated copper oxide nanowires for selective electrochemical reduction of aqueous CO2 to CO. J. Mater. Chem. A 2016, 4, 10710–10718. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Qin, B.; Wang, H.; Peng, F.; Yu, H.; Cao, Y. Effect of the surface roughness of copper substrate on three-dimensional tin electrode for electrochemical reduction of CO2 into HCOOH. J. CO2 Util. 2017, 21, 219–223. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Lv, W.; Fang, H.; Wang, W. Electrochemical reduction of CO2 to formate catalyzed by electroplated tin coating on copper foam. Appl. Surf. Sci. 2016, 362, 394–398. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J. Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts. Chem. Eng. J. 2016, 293, 161–170. [Google Scholar] [CrossRef]
- Chen, C.; Pang, Y.; Zhang, F.; Zhong, J.; Zhang, B.; Cheng, Z. Sharp Cu@Sn nanocones on Cu foam for highly selective and efficient electrochemical reduction of CO2 to formate. J. Mater. Chem. A 2018, 6, 19621–19630. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, J.; Kong, F.; Fang, H.; Wang, W. Porous tin-based film deposited on copper foil for electrochemical reduction of carbon dioxide to formate. Int. J. Hydrogen Energy 2016, 41, 1585–1591. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, J.; Zhang, X.; Lei, T.; Girma, A.; Liu, Y.; Zhang, J. Rational design and synthesis of SnOx electrocatalysts with coralline structure for highly improved aqueous CO2 reduction to formate. ChemElectroChem 2016, 3, 1618–1628. [Google Scholar] [CrossRef]
- Chen, Y.; Kanan, M.W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for Tin/Tin oxide thin-film catalysts. J. Am. Chem. Soc. 2012, 134, 1986–1989. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Jenndy; Yeo, B.S.; Seh, Z.W. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. Nat. Catal. 2018, 1, 922–934. [Google Scholar] [CrossRef]
- Cui, C.; Han, J.; Zhu, X.; Liu, X.; Wang, H.; Mei, D.; Ge, Q. Promotional effect of surface hydroxyls on electrochemical reduction of CO2 over SnOx/Sn electrode. J. Catal. 2016, 343, 257–265. [Google Scholar] [CrossRef]
- Cui, C.; Wang, H.; Zhu, X.; Han, J.; Ge, Q. A DFT study of CO2 electrochemical reduction on Pb(211) and Sn(112). Sci. China Chem. 2015, 58, 607–613. [Google Scholar] [CrossRef]
- Dutta, A.; Kuzume, A.; Rahaman, M.; Vesztergom, S.; Broekmann, P. Monitoring the chemical state of catalysts for CO2 electroreduction: An in operando study. ACS Catal. 2015, 5, 7498–7502. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Sun, Y.; Tang, Y.; Dai, L.; Hu, Q.; Fisher, A.; Yang, X.J. Facile synthesis of nanostructural high-performance Cu-Pb electrocatalysts for CO2 reduction. Adv. Mater. Interfaces 2019, 6, 1801200. [Google Scholar] [CrossRef]
- Wang, H.; Han, Z.; Zhang, L.; Cui, C.; Zhu, X.; Liu, X.; Han, J.; Ge, Q. Enhanced CO selectivity and stability for electrocatalytic reduction of CO2 on electrodeposited nanostructured porous Ag electrode. J. CO2 Util. 2016, 15, 41–49. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, M.; Zhang, S.; Liu, X.; Zhu, X.; Ge, Q.; Wang, H. Tuning Sn-Cu Catalysis for Electrochemical Reduction of CO2 on Partially Reduced Oxides SnOx-CuOx-Modified Cu Electrodes. Catalysts 2019, 9, 476. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050476
Li Q, Li M, Zhang S, Liu X, Zhu X, Ge Q, Wang H. Tuning Sn-Cu Catalysis for Electrochemical Reduction of CO2 on Partially Reduced Oxides SnOx-CuOx-Modified Cu Electrodes. Catalysts. 2019; 9(5):476. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050476
Chicago/Turabian StyleLi, Qianwen, Mei Li, Shengbo Zhang, Xiao Liu, Xinli Zhu, Qingfeng Ge, and Hua Wang. 2019. "Tuning Sn-Cu Catalysis for Electrochemical Reduction of CO2 on Partially Reduced Oxides SnOx-CuOx-Modified Cu Electrodes" Catalysts 9, no. 5: 476. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050476