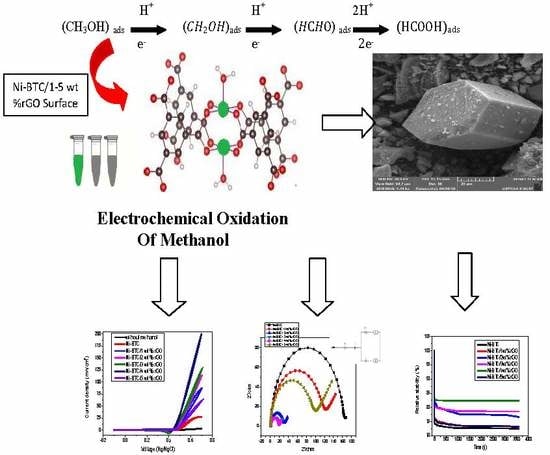
Development of Nickel-BTC-MOF-Derived Nanocomposites with rGO Towards Electrocatalytic Oxidation of Methanol and Its Product Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Measurements
2.2. Preparation of Working Electrodes
Bulk Electrolysis and Product Analysis
3. Material and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Ni-BTC-MOF
3.4. Synthesis of GO and rGO
3.5. Synthesis of Ni-BTC-MOF/rGO Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Armyanov, S.; Valova, E.; Avdeev, G.; Hubin, A.; Steenhaut, O.; Dille, J.; Tsiplakides, D.; Balomenou, S. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors. Appl. Catal. B Environ. 2013, 136, 160–167. [Google Scholar] [CrossRef]
- Appleby, A.J. Fuel Cell Handbook; Krieger Pub Co.: Malabar, FL, USA, 1989. [Google Scholar]
- Steele, B.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Bruno, M.M.; Williams, F.J.; Viva, F.A.; Corti, H.R. High-activity mesoporous Pt/Ru catalysts for methanol oxidation. ACS Appl. Mater. Interfaces 2013, 5, 10437–10444. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhang, Q.; Zhang, B.; Jin, Y.G.; Huang, J.Q.; Su, D.S.; Wei, F. Toward full exposure of “active sites”: Nanocarbon electrocatalyst with surface enriched nitrogen for superior oxygen reduction and evolution reactivity. Adv. Funct. Mater. 2014, 24, 5956–5961. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.; Zemlyanov, D.; Beloshapkin, S.; Knop-Gericke, A.; Schlögl, R.; Andrushkevich, T.; Bukhtiyarov, V. Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst. J. Catal. 2014, 311, 59–70. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Mathe, N.R.; Scriba, M.R.; Rikhotso, R.S.; Coville, N.J. Microwave-irradiation polyol synthesis of PVP-protected Pt–Ni electrocatalysts for methanol oxidation reaction. Electrocatalysis 2018, 9, 388–399. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Qiu, J.; Zhou, W.; Han, H.; Wei, Z.; Sun, G.; Xin, Q. Carbon nanotubes as support for cathode catalyst of a direct methanol fuel cell. Carbon 2002, 40, 787–790. [Google Scholar] [CrossRef]
- Mehek, R.; Iqbal, N.; Noor, T.; Nasir, H.; Mehmood, Y.; Ahmed, S. Novel Co-MOF/graphene oxide electrocatalyst for methanol oxidation. Electrochim. Acta 2017, 255, 195–204. [Google Scholar] [CrossRef]
- Heli, H.; Jafarian, M.; Mahjani, M.; Gobal, F. Electro-oxidation of methanol on copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Liu, F.; Lee, J.Y.; Zhou, W. Multi-Segment Pt–RuNi Nanorods for Methanol Electro-Oxidation at Room Temperature. J. Electrochem. Soc. 2006, 153, A2133–A2138. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, G.-R.; Wang, Z.-L.; Ou, Y.-N.; Tong, Y.-X. Pt nanorods aggregates with enhanced electrocatalytic activity toward methanol oxidation. J. Phys. Chem. C 2010, 114, 19175–19181. [Google Scholar] [CrossRef]
- Alia, S.M.; Zhang, G.; Kisailus, D.; Li, D.; Gu, S.; Jensen, K.; Yan, Y. Porous platinum nanotubes for oxygen reduction and methanol oxidation reactions. Adv. Funct. Mater. 2010, 20, 3742–3746. [Google Scholar] [CrossRef]
- Varela, F.R.; Savadogo, O. The effect of anode catalysts on the behavior of low temperature direct propane polymer electrolyte fuel cells (DPFC). J. New Mater. Electrochem. Syst. 2006, 9, 127. [Google Scholar]
- Cai, Z.; Martin, C.R. Electronically conductive polymer fibers with mesoscopic diameters show enhanced electronic conductivities. J. Am. Chem. Soc. 1989, 111, 4138–4139. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Yeh, C.-T. Poly (vinylpyrrolidone)-modified graphite carbon nanofibers as promising supports for PtRu catalysts in direct methanol fuel cells. J. Am. Chem. Soc. 2007, 129, 9999–10010. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Ci, L.; Wang, C.; Ajayan, P.M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 2010, 48, 1124–1130. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yeo, S.; Jeon, J.-D.; Kwak, S.-Y. Enhancement of hydrogen storage capacity and hydrostability of metal–organic frameworks (MOFs) with surface-loaded platinum nanoparticles and carbon black. Microporous Mesoporous Mater. 2015, 202, 8–15. [Google Scholar] [CrossRef]
- Khan, I.A.; Badshah, A.; Nadeem, M.A.; Haider, N.; Nadeem, M.A. A copper based metal-organic framework as single source for the synthesis of electrode materials for high-performance supercapacitors and glucose sensing applications. Int. J. Hydrogen Energy 2014, 39, 19609–19620. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Tang, H.; Cai, S.; Xie, S.; Wang, Z.; Tong, Y.; Pan, M.; Lu, X. Metal–organic-framework-derived dual metal- and nitrogen-doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Adv. Sci. 2016, 3, 1500265. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Dang, L.Q.; Nguyen, M.T.; Van Truong, N.; Le, P.H.; Long, N. Investigation of Carbon Supported Ru–Pt Nanoparticles for High–Performance Electrocatalytic Oxidation of Methanol. Int. J. Electrochem. Sci. 2017, 12, 10187–10198. [Google Scholar] [CrossRef]
- Behmenyar, G.; Akın, A.N. Investigation of carbon supported Pd–Cu nanoparticles as anode catalysts for direct borohydride fuel cell. J. Power Sources 2014, 249, 239–246. [Google Scholar] [CrossRef]
- Song, G.; Wang, Z.; Wang, L.; Li, G.; Huang, M.; Yin, F. Preparation of MOF (Fe) and its catalytic activity for oxygen reduction reaction in an alkaline electrolyte. Chin. J. Catal. 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Hanif, S.; Shi, X.; Iqbal, N.; Noor, T.; Anwar, R.; Kannan, A. ZIF derived PtNiCo/NC cathode catalyst for proton exchange membrane fuel cell. Appl.Catal. B Environ. 2019, 258, 117947. [Google Scholar] [CrossRef]
- Noor, T.; Zaman, N.; Nasir, H.; Iqbal, N.; Hussain, Z. Electro catalytic study of NiO-MOF/rGO composites for methanol oxidation reaction. Electrochim. Acta 2019, 307, 1–12. [Google Scholar] [CrossRef]
- Noor, T.; Ammad, M.; Zaman, N.; Iqbal, N.; Yaqoob, L.; Nasir, H. A Highly Efficient and Stable Copper BTC Metal Organic Framework Derived Electrocatalyst for Oxidation of Methanol in DMFC Application. Catal. Lett. 2019, 1–16. [Google Scholar] [CrossRef]
- Sarwar, E.; Noor, T.; Iqbal, N.; Mehmood, Y.; Ahmed, S.; Mehek, R. Effect of Co-Ni Ratio in Graphene Based Bimetallic Electro-catalyst for Methanol Oxidation. Fuel Cells 2018, 18, 189–194. [Google Scholar] [CrossRef]
- Rahim, M.A.; Hameed, R.A.; Khalil, M. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Sreeprasad, T.; Maliyekkal, S.M.; Lisha, K.; Pradeep, T. Reduced graphene oxide–metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.J. Graphene oxide nanopaint. Carbon 2014, 72, 328–337. [Google Scholar] [CrossRef]
- Zhou, L.; Kong, X.; Gao, M.; Lian, F.; Li, B.; Zhou, Z.; Cao, H. Hydrothermal fabrication of MnCO3@ rGO composite as an anode material for high-performance lithium ion batteries. Inorg. Chem. 2014, 53, 9228–9234. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF–graphite oxide composites: Combining the uniqueness of graphene layers and metal–organic frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- Israr, F.; Kim, D.K.; Kim, Y.; Oh, S.J.; Ng, K.C.; Chun, W. Cost effective and low energy consuming hydrothermal synthesis of Ni based MOF. J. Energ. Eng. 2015, 24, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Israr, F.; Chun, D.; Kim, Y.; Kim, D.K. High yield synthesis of Ni-BTC metal–organic framework with ultrasonic irradiation: Role of polar aprotic DMF solvent. Ultrason. Sonochem. 2016, 31, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.; Li, H.; Groy, T. Construction of porous solids from hydrogen-bonded metal complexes of 1, 3, 5-benzenetricarboxylic acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Jabarian, S.; Ghaffarinejad, A. Electrochemical Synthesis of NiBTC Metal Organic Framework Thin Layer on Nickel Foam: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1565–1574. [Google Scholar] [CrossRef]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical deposition of nickel graphene composite coatings: Effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef]
- Andrijanto, E.; Shoelarta, S.; Subiyanto, G.; Rifki, S. Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. In Proceedings of the AIP ICAMST 2015 Conference, Semarang, Indonesia, 6–7 October 2015. [Google Scholar]
- Zhang, Y.; Liu, H.; Zhu, Z.; Wong, K.-W.; Mi, R.; Mei, J.; Lau, W.-M. A green hydrothermal approach for the preparation of graphene/α-MnO2 3D network as anode for lithium ion battery. Electrochim. Acta 2013, 108, 465–471. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, Y.; Chen, L.; Xiong, P.; Wei, M. Hierarchical cerium oxide derived from metal-organic frameworks for high performance supercapacitor electrodes. Electrochim. Acta 2016, 222, 773–780. [Google Scholar] [CrossRef]
- Sun, K.; Li, L.; Yu, X.; Liu, L.; Meng, Q.; Wang, F.; Zhang, R. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 2017, 486, 128–135. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Li, S.; Zhang, J.; Yang, X.; Shen, P.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. 3D-monoclinic M–BTC MOF (M= Mn, Co, Ni) as highly efficient catalysts for chemical fixation of CO2 into cyclic carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303. [Google Scholar] [CrossRef]
- Hamidipour, L.; Farzaneh, F. Cobalt metal organic framework as an efficient heterogeneous catalyst for the oxidation of alkanes and alkenes. React. Kinet. Mech. Catal. 2013, 109, 67–75. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Hidayah, N.; Liu, W.-W.; Lai, C.-W.; Noriman, N.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 2013, 4, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolini, E.; Salgado, J.R.; Gonzalez, E.R. The methanol oxidation reaction on platinum alloys with the first row transition metals: The case of Pt–Co and–Ni alloy electrocatalysts for DMFCs: A short review. Appl. Catal. B Environ. 2006, 149, 137–149. [Google Scholar] [CrossRef]
- Cordeiro, C.; De Vries, M.; Cremers, T.; Westerink, B. The role of surface availability in membrane-induced selectivity for amperometric enzyme-based biosensors. Sens. Actuators B Chem. 2016, 223, 679–688. [Google Scholar] [CrossRef]
- Das, A.K.; Layek, R.K.; Kim, N.H.; Jung, D.; Lee, J.H. Reduced graphene oxide (RGO)-supported NiCo2O4 nanoparticles: An electrocatalyst for methanol oxidation. Nanoscale 2014, 6, 10657–10665. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, C.; Liu, J.; Wang, L.; Du, Y.; Qiao, S.-Z. Two-dimensional metal–organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 2017, 53, 10906–10909. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Parwaiz, S.; Bhunia, K.; Das, A.K.; Khan, M.M.; Pradhan, D. Cobalt-doped ceria/reduced graphene oxide nanocomposite as an efficient oxygen reduction reaction catalyst and supercapacitor material. J. Phys. Chem. C 2017, 121, 20165–20176. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Zhou, G.; Zhang, Z.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. A high-performance catalyst support for methanol oxidation with graphene and vanadium carbonitride. Nanoscale 2015, 7, 1301–1307. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K.; Reeve, R.W. A study of the anodic oxidation of methanol on Pt in alkaline solutions. J. Electroanal. Chem. 2003, 547, 17–24. [Google Scholar] [CrossRef]
- Niu, L.; Li, Q.; Wei, F.; Chen, X.; Wang, H. Electrochemical impedance and morphological characterization of platinum-modified polyaniline film electrodes and their electrocatalytic activity for methanol oxidation. J. Electroanal. Chem. 2003, 544, 121–128. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, P.; Xu, S.; Yan, X.; Xue, Q. Free-standing three-dimensional graphene/manganese oxide hybrids as binder-free electrode materials for energy storage applications. ACS Appl. Mater. Interfaces 2014, 6, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, M.; Zhang, X.; Cai, J.; Lin, S. A novel octahedral MnO/RGO composite prepared by thermal decomposition as a noble-metal free electrocatalyst for ORR. J. Mater. Sci. 2017, 52, 6656–6669. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, H. Tin oxide nanoparticle-modified commercial PtRu catalyst for methanol oxidation. Micro Nano Lett. 2013, 8, 23–26. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Liu, M.; Guo, Y. Fabrication of a novel PtPbBi/C catalyst for ethanol electro-oxidation in alkaline medium. Electrochim. Acta 2012, 83, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Da, H.; Wang, R.; Ji, S. Beef-derived mesoporous carbon as highly efficient support for PtRuIr electrocatalysts and their high activity for CO and methanol oxidation. S. Afr. J. Chem. 2014, 67, 33–39. [Google Scholar]
- Ye, W.; Zhang, X.; Chen, Y.; Du, Y.; Zhou, F.; Wang, C. Pulsed electrodeposition of reduced graphene oxide on glass carbon electrode as an effective support of electrodeposited Pt microspherical particles: Nucleation studies and the application for methanol electro-oxidation. Int. J. Electrochem. Sci. 2013, 8, e2139. [Google Scholar]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum–nickel hydroxide–graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, H.; Dai, Y.; Zhang, N.; Zhao, W.; Wang, S.; Lou, Y.; Li, Y.; Sun, Y. Preparation and characterization of Pt/TiO2 nanofibers catalysts for methanol electro-oxidation. Electrochim. Acta 2015, 178, 74–79. [Google Scholar] [CrossRef]
- Vigier, F.; Rousseau, S.; Coutanceau, C.; Leger, J.-M.; Lamy, C. Electrocatalysis for the direct alcohol fuel cell. Top. Catal. 2006, 40, 111–121. [Google Scholar] [CrossRef]
- Asiri, H.A.; Anderson, A.B. Mechanisms for ethanol electrooxidation on Pt (111) and adsorption bond strengths defining an ideal catalyst. J. Electrochem. Soc. 2015, 162, F115–F122. [Google Scholar] [CrossRef]
- Yang, Y.; McElwee-White, L. Electrochemical oxidation of methanol using dppm-bridged Ru/Pd, Ru/Pt and Ru/Au catalysts. Dalton Trans. 2004, 15, 2352–2356. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.-F.; Guo, S.-X.; Bond, A.M.; Zhang, J.; Zhu, G.; Hill, C.L.; Geletii, Y.V. Electrooxidation of Ethanol and Methanol Using the Molecular Catalyst [{Ru4O4 (OH) 2 (H2O) 4}(γ-SiW10O36) 2]10−. J. Am. Chem. Soc. 2016, 138, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Nahm, C.; Kim, C.; Park, Y.; Park, B. Nanoporous Pt thin films with superior catalytic activities by the electrochemical dissolution of Al. Met. Mater. Int. 2009, 15, 989–992. [Google Scholar] [CrossRef]
- Nguyen, T.; Thuy, V.; Luu, C.L.; Hoang, T.C.; Nguyen, T.; Bui, T.H.; Duy, N.; Phuc, H.; Pham, T.; Thuy, P. Synthesis of MOF-199 and application to CO2 adsorption. Adv. Nat. Sci. Nanosci. Nanotechnol. (Online) 2013, 4. [Google Scholar] [CrossRef]




















| Sample Element | Ni-BTC | Ni-BTC/1 wt % rGO | Ni-BTC/2 wt % rGO | Ni-BTC/3 wt % rGO | Ni-BTC/4 wt % rGO | Ni-BTC/5 wt % rGO |
|---|---|---|---|---|---|---|
| C wt % | 35.66 | 39.66 | 42.13 | 45.65 | 53.11 | 53.69 |
| O wt % | 26.89 | 29.05 | 36.99 | 41.88 | 39.67 | 41.06 |
| Ni wt % | 37.45 | 31.29 | 20.92 | 12.48 | 7.22 | 5.25 |
| Catalyst | R2 | Diffusion Co-Efficient (cm2/s) |
|---|---|---|
| Ni-BTC | 0.99256 | 1.643 × 10−5 |
| Ni-BTC/1 wt % rGO | 0.98716 | 15.35 × 10−5 |
| Ni-BTC/2 wt % rGO | 0.99269 | 23.60 × 10−5 |
| Ni-BTC/3 wt % rGO | 0.98708 | 32.74 × 10−5 |
| Ni-BTC/4 wt % rGO | 0.99833 | 84.57 × 10−5 |
| Ni-BTC/5 wt % rGO | 0.99184 | 7.494 × 10−5 |
| Sr.No | Sample | Resistance (Rs) Ohm | Resistance (Rct) Ohm | Capacitance (C) Farad |
|---|---|---|---|---|
| 1 | Ni-BTC | 1.340 | 158.00 | 6.209e−6 |
| 2 | Ni-BTC/1 wt % rGO | 0.624 | 112.90 | 8.316e−6 |
| 3 | Ni-BTC/2 wt % rGO | 0.663 | 28.23 | 1.444e−5 |
| 4 | Ni-BTC/3 wt % rGO | 0.464 | 19.12 | 1.229e−5 |
| 5 | Ni-BTC/4 wt % rGO | 0.328 | 18.12 | 9.156e−6 |
| 6 | Ni-BTC/5 wt % rGO | 0.496 | 102.00 | 4.728e−6 |
| Catalyst | Rct (Ohm) | Tafel Slope (mV/dec) |
|---|---|---|
| Ni-BTC | 158 | 59.65 |
| Ni-BTC/1 wt % rGO | 112 | 33.69 |
| Ni-BTC/2 wt % rGO | 28.23 | 29.58 |
| Ni-BTC/3 wt % rGO | 19.12 | 28.10 |
| Ni-BTC/4 wt % rGO | 18.12 | 27.89 |
| Ni-BTC/5 wt % rGO | 102 | 75.47 |
| Catalytic Materials | Methanol Concentration (M) | Catalyst Amount (mg/cm2) | Scan Rate (mV/s) | Anodic Potential (V) | Peak Current Density (mA/cm2) | Resistance (Ohm) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt-Ni | 1 | 20 | 50 | 1.44 vs. RHE | 265.6 | - | [9] |
| Ru@Pt/MWCNT | 2 | 4 | 50 | 1.81 vs. RHE | 182.4 | 606 | [29] |
| NiCo2O4-rGO | 0.5 | - | 50 | 1.26 vs. RHE | 48.0 | 1000 | [52] |
| Nanoporous Pt/Al thin films | 2 | - | 100 | 0.8 vs. RHE | 6.0 | - | [76] |
| Ni-BTC | 2 | 1.07 | 50 | 1.66 vs. RHE | 27.155 | 158 | This work |
| Ni-BTC/1 wt % rGO | 2 | 1.07 | 50 | 1.66 vs. RHE | 86.346 | 112 | This work |
| Ni-BTC/2 wt % rGO | 2 | 1.07 | 50 | 1.66 vs. RHE | 112.04 | 28.23 | This work |
| Ni-BTC/3 wt % rGO | 2 | 1.07 | 50 | 1.66 vs. RHE | 125.83 | 19.12 | This work |
| Ni-BTC/4 wt % rGO | 2 | 1.07 | 50 | 1.66 vs. RHE | 200.02 | 18.12 | This work |
| Ni-BTC/5 wt % rGO | 2 | 1.07 | 50 | 1.66 vs. RHE | 60.72 | 102 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Zaman, N. Development of Nickel-BTC-MOF-Derived Nanocomposites with rGO Towards Electrocatalytic Oxidation of Methanol and Its Product Analysis. Catalysts 2019, 9, 856. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100856
Yaqoob L, Noor T, Iqbal N, Nasir H, Zaman N. Development of Nickel-BTC-MOF-Derived Nanocomposites with rGO Towards Electrocatalytic Oxidation of Methanol and Its Product Analysis. Catalysts. 2019; 9(10):856. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100856
Chicago/Turabian StyleYaqoob, Lubna, Tayyaba Noor, Naseem Iqbal, Habib Nasir, and Neelam Zaman. 2019. "Development of Nickel-BTC-MOF-Derived Nanocomposites with rGO Towards Electrocatalytic Oxidation of Methanol and Its Product Analysis" Catalysts 9, no. 10: 856. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100856