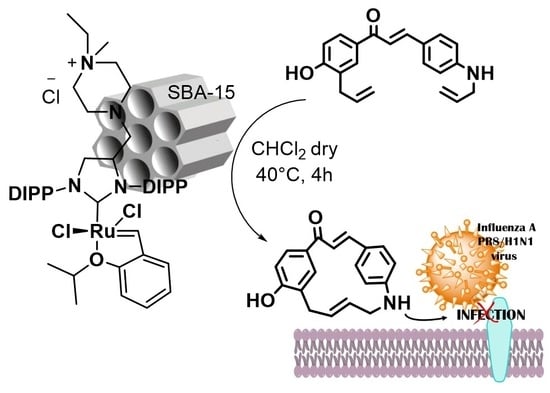
Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis
Abstract
:1. Introduction
2. Results
2.1. ASIPr/SBA-15 Catalyzed Synthesis of Stilbene Derivatives
2.2. Antioxidant Activity of Compounds 8–14 and 17–23
2.3. Antiviral Activity Against Influenza A Virus Replication
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Textural Parameters
3.4. ICP–MS Analysis
3.5. Synthesis of Compounds 8–14, 17–19 and 22–23
3.5.1. (E)-4,4’-(but-2-ene-1,4-diyl)bis(2-methoxyphenol) (8)
3.5.2. (E)-4,4’-(but-2-ene-1,4-diyl)bis(2,6-dimethoxyphenol) (9)
3.5.3. (E)-5,5’-(but-2-ene-1,4-diyl)bis(3-methoxybenzene-1,2-diol) (10)
3.5.4. (E)-1,1’-(but-2-ene-1,4-diylbis(4-hydroxy-3,1-phenylene))bis(ethan-1-one) (11)
3.5.5. (E)-2,2’-(but-2-ene-1,4-diyl)diphenol (12)
3.5.6. (E)-4,4’-(ethene-1,2-diyl)bis(2-methoxyphenol) (13)
3.5.7. (E)-5,5’-(ethene-1,2-diyl)bis(3-methoxybenzene-1,2-diol) (14)
3.5.8. (E)-5-(4-(4-hydroxy-3,5-dimethoxyphenyl)but-2-en-1-yl)-3-methoxybenzene-1,2-diol (17)
3.5.9. (E)-3-(4-(4-hydroxy-3, 5-dimethoxyphenyl)but-2-en-1-yl)benzene-1,2-diol (18)
3.5.10. (E)-3-(4-(2-hydroxyphenyl)but-2-en-1-yl)benzene-1,2-diol (19)
3.5.11. (3E,8E)-14-hydroxy-6-aza-1(1,3),5(1,4)-dibenzenacyclodecaphane-3,8-dien-2-one (22)
3.5.12. (3E,8E)-14-hydroxy-6-oxa-1(1,3),5(1,4)-dibenzenacyclodecaphane-3,8-dien-2-one (23)
3.6. Synthesis of (E)-1-(3-allyl-4-hydroxyphenyl)-3-(4-(allylamino)phenyl)prop-2-en-1-one (20)
3.7. Synthesis of (E)-1-(3-allyl-4-hydroxyphenyl)-3-(4-fluorophenyl)prop-2-en-1-one
3.8. Synthesis of Compound 21
3.9. Synthesis of 4-(Allyloxy)Benzaldehyde
3.10. Evaluation of Antioxidant Activity by DPPH Assay
3.11. Virus Production and Infection
3.12. Haemagglutination (HAU) Assay
3.13. In Cell Western (ICW) Assay
3.14. RT-PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaw, M.L.; Palese, P. Orthomyxoviridae: The Viruses and Their Replication. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1648–1698. [Google Scholar]
- Go, Y.-M.; Kang, S.-M.; Roede, J.R.; Orr, M.; Jones, D.P. Increased Inflammatory Signaling and Lethality of Influenza H1N1 by Nuclear Thioredoxin-1. PLoS ONE 2011, 6, e18918. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, L.; Aquilano, K.; Cozzolino, F.; Garaci, E.; Palamara, A.T.; Iuvara, A.; Ciriolo, M.R.; Rotilio, G. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Gannagé, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Rämer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G.; et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Sgarbanti, R.; Amatore, D.; Celestino, I.; Marcocci, M.E.; Fraternale, A.; Ciriolo, M.R.; Magnani, M.; Saladino, R.; Garaci, E.; Palamara, A.T.; et al. Intracellular redox state as target for anti-influenza therapy: Are antioxidants always effective? Curr. Top. Med. Chem. 2014, 14, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Jian, W.; He, D.; Song, S. Synthesis, Biological Evaluation and Molecular Modeling Studies of New Oxadiazole-Stilbene Hybrids against Phytopathogenic Fungi. Sci. Rep. 2016, 6, 31045. [Google Scholar] [CrossRef]
- Mahboub, R.; Memmou, F. Antioxidant activity and kinetics studies of eugenol and 6-bromoeugenol. Nat. Prod. Res. 2014, 29, 966–971. [Google Scholar] [CrossRef]
- Rimando, A.; Suh, N. Biological/Chemopreventive Activity of Stilbenes and their Effect on Colon Cancer. Planta Med. 2008, 74, 1635–1643. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Li, J.; Liu, J.; Cha, L.; Mu, J. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clin. Exp. Hypertens. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y. Pterostilbene, a novel natural plant conduct, inhibits high fat induced atherosclerosis inflammation via nf-κb signaling pathway in toll-like receptor 5(tlr5) deficient mice. Biomed. Pharmacother. 2016, 81, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pu, H.; Hu, C. Neuroprotection by combination of resveratrol and enriched environment against ischemic brain injury in rats. Neurol. Res. 2016, 38, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Baile, C.A.; Yang, J.-Y.; Rayalam, S.; Hartzell, D.L.; Lai, C.-Y.; Andersen, C.; Della-Fera, M.A.; Della-Fera, M.A. Effect of resveratrol on fat mobilization. Ann. N. Y. Acad. Sci. 2011, 1215, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Nealon, R.; Scholey, A.; Howe, P. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial Activity of Resveratrol Analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef]
- Campagna, M.; Rivas, C. Antiviral activity of resveratrol. Biochem. Soc. Trans. 2010, 38, 50–53. [Google Scholar] [CrossRef]
- Benencia, F.; Courrèges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495500. [Google Scholar] [CrossRef]
- Liu, T.; Zang, N.; Zhou, N.; Li, W.; Xie, X.; Deng, Y.; Ren, L.; Long, X.; Li, S.; Zhou, L.; et al. Resveratrol Inhibits the TRIF-Dependent Pathway by Upregulating Sterile Alpha and Armadillo Motif Protein, Contributing to Anti-Inflammatory Effects after Respiratory Syncytial Virus Infection. J. Virol. 2014, 88, 4229–4236. [Google Scholar] [CrossRef]
- Heredia, A.; Davis, C.; Amin, M.N.; Le, N.M.; Wainberg, M.A.; Oliveira, M.; Deeks, S.G.; Wang, L.-X.; Redfield, R.R. Targeting host nucleotide biosynthesis with resveratrol inhibits emtricitabine-resistant HIV-1. AIDS 2014, 28, 317–323. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Gu, Z.; Wang, Y.; Shi, M.; Ji, Y.; Sun, J.; Xu, X.; Zhang, L.; Jiang, J.; et al. Resveratrol Inhibits Enterovirus 71 Replication and Pro-Inflammatory Cytokine Secretion in Rhabdosarcoma Cells through Blocking IKKs/NF-κB Signaling Pathway. PLoS ONE 2015, 10, 116879. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Saito, H.; Ikeda, M.; Hokari, R.; Kato, N.; Hibi, T.; Miura, S. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J. Gastroenterol. 2010, 16, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Hernandez, L.; Cozzolino, F.; Palamara, A.T.; Nencioni, L.; De Chiara, G.; Ciriolo, M.R.; Garaci, E. Inhibition of Influenza A Virus Replication by Resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar]
- Grubbs, R.H. Handbook of Metathesis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2003; ISBN 978-3-527-30616-9. [Google Scholar]
- Grubbs, R.H.; Trnka, T.M. Ruthenium-Catalyzed Olefin Metathesis. In Ruthenium in Organic Synthesis; Murahashi, S., Ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 153–177. [Google Scholar]
- Bilel, H.; Hamdi, N.; Zagrouba, F.; Fischmeister, C.; Bruneau, C. Eugenol as a renewable feedstock for the production of polyfunctional alkenes via olefin cross-metathesis. RSC Adv. 2012, 2, 9584. [Google Scholar] [CrossRef]
- Blackwell, H.E.; O’Leary, D.J.; Chatterjee, A.K.; Washenfelder, R.A.; Bussmann, D.A.; Grubbs, R.H. New approaches to olefin cross-metathesis. J. Am. Chem. Soc. 2000, 122, 58–71. [Google Scholar] [CrossRef]
- Vieille-Petit, L.; Clavier, H.; Linden, A.; Blumentritt, S.; Nolan, S.P.; Dorta, R. Ruthenium olefin metathesis catalysts with N-heterocyclic carbene ligands bearing n-naphthyl side chains. Organometallics 2010, 29, 775. [Google Scholar] [CrossRef]
- De Souza, T.B.; De Oliveira Brito, K.M.; Silva, N.C.; Rocha, R.P.; De Sousa, G.F.; Duarte, L.P.; Coelho LF, L.; Dias AL, T.; Veloso, M.P.; Carvalho, D.T.; et al. New Eugenol Glucoside-Based Derivative Shows Fungistatic and Fungicidal Activity against Opportunistic Candida Glabrata. Chem. Biol. Drug Des. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Broggi, J.; Urbina-Blanco, C.A.; Clavier, H.; Leitgeb, A.; Slugovc, C.; Slawin AM, Z.; Nolan, S.P. The Influence of Phosphane Ligands on the Versatility of Ruthenium–Indenylidene Complexes in Metathesis. Chem. Eur. J. 2010, 16, 9215. [Google Scholar] [CrossRef]
- Moıse, J.; Arseniyadis, S.; Cossy, J. Cross-Metathesis between α-Methylene-γ-butyrolactone and Olefins: A Dramatic Additive Effect. Org. Lett. 2007, 9, 1695–1698. [Google Scholar] [CrossRef]
- Harvey, B.G.; Sahagun, C.M.; Guenthner, A.J.; Groshens, T.J.; Cambrea, L.R.; Reams, J.T.; Mabry, J.M. A High-Performance Renewable Thermosetting Resin Derived from Eugenol. ChemSusChem 2014, 7, 1964–1969. [Google Scholar] [CrossRef]
- Hasib-Ur-Rahman, M.; Belkacemi, K.; Hamoudi, S. Heterogeneous olefin-metathesis: Comparative perspective of the activity with respect to unsaturated fatty acid methyl esters. Can. J. Chem. Eng. 2017, 95, 1850–1863. [Google Scholar] [CrossRef]
- Suriboot, J.; Bazzi, H.S.; Bergbreiter, D.E. Supported Catalysts Useful in Ring-Closing Metathesis, Cross Metathesis, and Ring-Opening Metathesis Polymerization. Polymers 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öztürk, B.G. Ammonium tagged Hoveyda-Grubbs catalysts immobilized on magnetically separable core-shell silica supports for ring-closing metathesis reactions. Microporous Mesoporous Mater. 2018, 267, 249–256. [Google Scholar] [CrossRef]
- Bhar, S.; Ananthakrishnan, R. Ru(ii)-Metal complex immobilized mesoporous SBA-15 hybrid for visible light induced photooxidation of chlorophenolic compounds in aqueous medium. Photochem. Photobiol. Sci. 2017, 16, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, J.; Li, Q.; Stucky, G.D. Morphological Control of Highly Ordered Mesoporous Silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Pastva, J.; Skowerski, K.; Czarnocki, S.J.; Žilková, N.; Čejka, J.; Bastl, Z.; Balcar, H. Ru-Based Complexes with Quaternary Ammonium Tags Immobilized on Mesoporous Silica as Olefin Metathesis Catalysts. ACS Catal. 2014, 4, 3227–3236. [Google Scholar] [CrossRef]
- Balcar, H.; Čejka, J. Mesoporous molecular sieves as advanced supports for olefin metathesis catalysts. Coord. Chem. Rev. 2013, 257, 3107–3124. [Google Scholar] [CrossRef]
- Liao, Z.-H.; Hsu, P.-W.; Hung, T.-C.; Liao, G.-J.; Chern, Z.-Y.; Lai, Y.-L.; Yu, L.-C.; Hsu, Y.-J.; Wang, J.-H.; Chen, P.; et al. Investigation of Thermal Stability and Reactivity of Rh Nanoclusters on an Ultrathin Alumina Film. Catalysts 2019, 9, 971. [Google Scholar] [CrossRef] [Green Version]
- Balcar, H.; Žilková, N.; Kubů, M.; Polášek, M.; Zedník, J. Metathesis of cardanol over ammonium tagged Hoveyda-Grubbs type catalyst supported on SBA-15. Catal. Today 2018, 304, 127–134. [Google Scholar] [CrossRef]
- Delaude, L.; Demonceau, A.; Noels, A.F.; Ferré-Filmon, K.; Ferre-Filmon, K. Stereoselective Synthesis of (E)-Hydroxystilbenoids by Ruthenium-Catalyzed Cross-Metathesis. Eur. J. Org. Chem. 2005, 2005, 3319–3325. [Google Scholar]
- Krzysztof, S.; Lukasz, G.; Micha, B.; Czarnocki, S.; Szczepaniak, G.; Wierzbicka, C. (4-((4-Ethyl-4-methylpiperazin-1-ium-1-yl)methyl)-1,3-dimesitylimidazolidin-2 ylidene)dichloro(2-isopropoxybenzylidene)ruthenium(II) chloride. Patent Number PCT/EP2013/053967, 6 September 2013. [Google Scholar]
- Bannin, T.J.; Datta, P.P.; Kiesewetter, E.T.; Kiesewetter, M.K. Synthesizing Stilbene by Olefin Metathesis Reaction Using Guided Inquiry to Compare and Contrast Wittig and Metathesis Methodologies. J. Chem. Educ. 2019, 96, 143–147. [Google Scholar] [CrossRef]
- Lo, C.H.; Cariou, R.; Fischmeister, C.; Dixneufa, P.H. Styrene Initiators. Arab. J. Chem. 2016, 9, 931–935. [Google Scholar]
- Fioravanti, R.; Celestino, I.; Costi, R.; Crucitti, G.C.; Pescatori, L.; Mattiello, L.; Novellino, E.; Checconi, P.; Palamara, A.T.; Nencioni, L.; et al. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorganic Med. Chem. 2012, 20, 5046–5052. [Google Scholar] [CrossRef] [PubMed]
- Siatka, T.; Kašparová, M. Seasonal Variation in Total Phenolic and Flavonoid Contents and DPPH Scavenging Activity of Bellis perennis L. Flowers. Molecules 2010, 15, 9450–9461. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, B.M.; Botta, L.; Capecchi, E.; Celestino, I.; Checconi, P.; Palamara, A.T.; Nencioni, L.; Saladino, R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-influenza Activities. J. Nat. Prod. 2017, 80, 3247–3254. [Google Scholar] [CrossRef]
- Fujisawa, S.; Murakami, Y. Eugenol and Its Role in Chronic Diseases; Springer Science and Business Media LLC: Berlin, Germany, 2016; Volume 929, pp. 45–66. [Google Scholar]
- Wan, Y.; Zhou, Z.; Yang, Y.; Wang, J.; Hung, T. Application of an In-Cell Western assay for measurement of influenza A virus replication. J. Virol. Methods 2010, 169, 359–364. [Google Scholar] [CrossRef]







| BET Surface Area (m2/g) | Void Volume (cm3/g) | Average Pore Diameter (nm) | |
|---|---|---|---|
| SBA-15 | 861 | 1.12 | 5.5 |
| ASIPr/SBA-15 | 693 | 0.98 | 5.4 |
| Entry | Substrate(s) | Catalyst | Conversion (%) | Product | Yield (%) c |
|---|---|---|---|---|---|
| 1 | 1 | ASIPr | 71 | 8 | 48 |
| 2 | 1 | ASIPr/SBA-15 | 70 | 8 | 52 |
| 3 | 2 | ASIPr/SBA-15 | 75 | 9 | 56 |
| 4 | 3 | ASIPr/SBA-15 | 68 | 10 | 41 |
| 5 | 4 | ASIPr/SBA-15 | 35 | 11 | 18 |
| 6 | 5 | ASIPr/SBA-15 | 27 | 12 | 15 |
| 7 | 6 | ASIPr | 88 | 13 | 70 |
| 8 | 6 | ASIPr/SBA-15 | 93 | 13 | 73 |
| 9 | 7 | ASIPr/SBA-15 | 95 | 14 | 75 |
| 10 | 2, 3 b | ASIPr | 91 | 17(9) c(10) c | 68(12)(9) |
| 11 | 2, 3 b | ASIPr/SBA-15 | 90 | 17(9) c(10) c | 65 (11)(10) |
| 12 | 2, 15 b | ASIPr/SBA-15 | 45 | 18(9) c | 25(16) |
| 13 | 5, 15 b | ASIPr/SBA-15 | 30 | 19(12) c | 22(4) |
| 14 | 20 | ASIPr | 60 | 22 | 21 |
| 15 | 20 | ASIPr/SBA-15 | 58 | 22 | 24 |
| 16 | 21 | ASIPr/SBA-15 | 55 | 23 | 21 |
| Entry | Substrate | Conversion (%) | Yield (%) | Ru Leaching b |
|---|---|---|---|---|
| 1 | 2 | 73 | 56 | 1.6 |
| 2 | 2 | 72 | 56 | 1.4 |
| 3 | 2 | 70 | 52 | 1.0 |
| 4 | 2 | 70 | 51 | 1.2 |
| 5 | 2 | 64 | 47 | 1.3 |
| 6 | 2 | 60 | 46 | 1.1 |
| DPPH Assay | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Compound | % Inhibition | IC50 a | ||||
| 500 | 250 | 100 | 50 | 10 | |||
| 1 | A.A. | 99.9 | 97.1 | 92.4 | 79.19 | 4.4 | 13.2 |
| 2 | eugenol | 99.9 | 97.1 | 92.4 | 75.19 | 38.4 | 19.6 |
| 3 | 8 | 98.1 | 91.6 | 83.7 | 60.2 | 26.8 | 35.4 |
| 4 | 9 | 98.2 | 91.8 | 83.9 | 60.4 | 26.9 | 34.9 |
| 5 | 10 | 98.2 | 96.1 | 87.4 | 67.1 | 30.7 | 31.3 |
| 6 | 11 | 95.3 | 85.2 | 67.1 | 47.3 | 13.0 | 54.6 |
| 7 | 12 | 96.1 | 86.6 | 71.0 | 48.3 | 16.1 | 52.1 |
| 8 | 13 | 96.5 | 90.5 | 82.2 | 59.5 | 28.4 | 36.4 |
| 9 | 14 | 98.0 | 91.4 | 83.5 | 58.7 | 26.9 | 35.2 |
| 10 | 19 | 91.1 | 88.2 | 75.4 | 56.2 | 16.4 | 40.1 |
| 11 | 20 | 95.1 | 89.9 | 78.6 | 53.5 | 18.4 | 45.3 |
| 12 | 21 | 86.11 | 72.2 | 55.9 | 33.0 | 8.1 | 81.3 |
| 13 | 22 | 83.1 | 69.9 | 50.1 | 28.4 | 2.7 | 98.3 |
| 14 | 23 | 84.3 | 70.2 | 53.6 | 31.1 | 5.6 | 90.1 |
| Entry | Compound | IC50 a | SI b |
|---|---|---|---|
| 1 | 8 | 45.2 | 0.9 |
| 2 | 9 | 14.6 | n.a. c |
| 3 | 10 | 185.4 | 1.4 |
| 4 | 11 | 55 | 0.8 |
| 5 | 12 | 7.9 | 1.4 |
| 6 | 13 | 29.1 | 0.8 |
| 7 | 14 | 31.6 | 2.3 |
| 8 | 17 | 30.2 | 1.9 |
| 9 | 18 | 174 | n.a. |
| 10 | 19 | n.a. | n.a. |
| 11 | 20 | 29.7 | 1.1 |
| 12 | 21 | 37.1 | 0.4 |
| 13 | 22 | 9.5 | 4.4 |
| 14 | 23 | 24.5 | 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzarri, B.M.; Fanelli, A.; Piccinino, D.; De Angelis, M.; Dolfa, C.; Palamara, A.T.; Nencioni, L.; Zippilli, C.; Crucianelli, M.; Saladino, R. Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis. Catalysts 2019, 9, 983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9120983
Bizzarri BM, Fanelli A, Piccinino D, De Angelis M, Dolfa C, Palamara AT, Nencioni L, Zippilli C, Crucianelli M, Saladino R. Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis. Catalysts. 2019; 9(12):983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9120983
Chicago/Turabian StyleBizzarri, Bruno Mattia, Angelica Fanelli, Davide Piccinino, Marta De Angelis, Camilla Dolfa, Anna Teresa Palamara, Lucia Nencioni, Claudio Zippilli, Marcello Crucianelli, and Raffaele Saladino. 2019. "Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis" Catalysts 9, no. 12: 983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9120983