1. Introduction
The mechanical stretch of cardiac myocytes activates NADPH oxidase 2 (NOX2) which generates reactive oxygen species (ROS) signals that target ryanodine receptor type 2 (RyR2) to activate Ca
2+ spark rate [
1,
2]. While this mechano-chemo transduction pathway, known as X-ROS signaling, is providing a new understanding of cardiomyocyte function in health and disease [
1,
2,
3,
4,
5,
6], the molecular details of this pathway remain to be fully defined.
We have developed a computational model to dissect the mechanism of X-ROS signaling and predict the impact it has on normal and pathological physiology [
5]. One prediction of the model was that that stretching just prior to the action potential, such as that which might occur by the filling of the ventricles, optimally potentiates action potential-induced Ca
2+ release. In fact, in cardiac myocytes, stretching has been shown to increase the magnitude of the Ca
2+ and force transients during pacing [
7,
8].
Troponin C (TnC) is the Ca
2+ binding subunit of the troponin complex that is also comprised of an inhibitory subunit, troponin I (TnI) and a tropomyosin binding subunit, troponin T (TnT). TnC has two COOH-terminal high-affinity Ca
2+ binding sites (site III and IV) that can also bind Mg
2+ (also known as Ca
2+-Mg
2+ sites) and two NH
2-terminal low-affinity Ca
2+ binding sites (site I and II) that are Ca
2+-specific. Only the Ca
2+-specific sites in TnC can trigger muscle contraction [
9,
10] and in the case of cardiac TnC, only site II can initiate muscle contraction [
11,
12,
13].
A reduction in force or sarcomere length reduces the Ca
2+ sensitivity of cardiac troponin C (cTnC) [
14]. The changes in Ca
2+ sensitivity have been attributed to a change in the number of actin–myosin cross-bridges, which may arise from length-dependent changes in the spacing between actin and myosin filaments as well as structural rearrangements in the thin and thick filaments [
15,
16]. An increase in sarcomere length decreases the separation between actin and myosin and causes a change in troponin structure.
In this manuscript, we pursue an integrative experimental and computational modeling approach to improve our understanding of X-ROS signaling and its effect on excitation–contraction coupling by considering length-dependent changes in troponin Ca2+ sensitivity. During systole, the sarcoplasmic reticulum (SR) releases Ca2+ ions that bind to troponin C (TnC) present on actin (the thin filament). The binding of Ca2+ ions to TnC changes the conformation of TnC, exposes myosin binding sites on actin and thus initiates myocyte contraction. Crossbridge formation between actin and myosin increases the Ca2+ binding affinity of TnC and contributes to increasing the force of contraction of the cardiac myocytes.
The combination of experiments and modeling suggest that stretching offers two antagonistic effects on the level of measured [Ca2+]i during excitation–contraction coupling. Stretching increases the affinity of troponin for Ca2+, increasing Ca2+ buffering and force generation, i.e., buffering by troponin promotes the contraction. On the other hand, X-ROS signaling provides an increase in Ca2+ mobilization during stretching that contributes to increases in the measured [Ca2+]i level and hence may contribute to contraction. It does this by offsetting a possible reduction in measured [Ca2+]i due to the buffering of Ca2+ by troponin.
3. Results
Informed by our previously published results, we updated our model and performed numerical experiments using model simulations to isolate and dissect the mechanisms of stretching-dependent Troponin C Ca
2+ affinity on EC coupling. Similar to our previous experimental findings [
1], this improved model of X-ROS signaling produces a ~two-fold increase in Ca
2+ spark frequency upon myocyte stretch (
Figure 2A) that resolves to baseline when returned to resting length. Additionally, the model simulation shows a very small, transient rise in the myoplasmic Ca
2+ concentration ([Ca
2+]
i) (
Figure 2B) as we previously showed experientially [
5]. Model simulations revealed the contribution of Troponin C buffering (
Figure 2C) occurred independent of length-dependent changes in the affinity of troponin for Ca
2+.
The next set of numerical experiments interrogated the previous report of a 20% increase in troponin C Ca
2+ affinity driven by a 10% increase in length [
18]. Here, we observed that with an acute stretching, Ca
2+ spark frequency increases abruptly by ~1.7-fold which closely recapitulates our experimental data [
1] (
Figure 2D). While stretching also elicits an abrupt increase in troponin Ca
2+ sensitivity which predisposes a sudden decrease in [Ca
2+]
i, the simultaneous increase in X-ROS elicits Ca
2+ sparks, which offsets the effect (
Figure 2E). Furthermore, when the myocyte length is returned to resting length, the abrupt decrease in the troponin sensitivity drives a transient elevation of [Ca
2+]
i, which is offset by the immediate decrease in Ca
2+ spark frequency. Consistent with this behavior is the numerical result of a sustained stretch, which would maintain the increased affinity of Troponin for Ca
2+ [Ca
2+]
trpn throughout the stretched period (
Figure 2F).
Cardiomyocyte stretching therefore activates at least two mechanisms: (1) X-ROS signaling that increases SR Ca
2+ release (via spark activation,
Figure 2A) resulting in increased [Ca
2+]
i (
Figure 2B), and (2) an increase in the Ca
2+ binding affinity of troponin which increases Ca
2+ buffering. To extend these concepts to the global Ca
2+ transient, experiments were performed in electrically stimulated myocytes.
Figure 3A shows experiments in cardiomyocytes field stimulated at 1 Hz in which the peak Ca
2+ concentration (ΔF/F
0) was quantified before, during, and after, stretching to one-half of the cardiomyocyte. Transients within the 10 s duration of each period were averaged and normalized to the period before stretching (
Figure 3E). Here, we show that stretching elicited a small rise in the peak [Ca
2+]
i of the stretched (
Figure 3A) vs. non-stretched (
Figure 3D) region that was rapidly reversed upon return to its unstretched level. Given that actin–myosin cross-bridge formation is required for the length-dependent change in TnC Ca
2+ sensitivity [
19], we sought to disrupt cross-bridge formation as an empirical test of Troponin C’s effect.
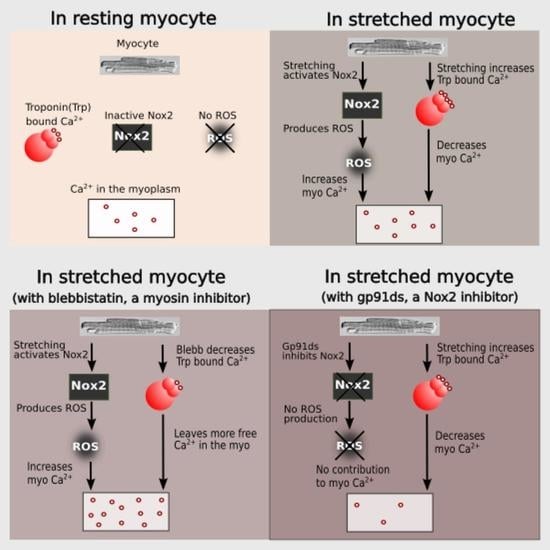
Blebbistatin is a chemical that inhibits actin and myosin crossbridge formation by lowering the affinity of myosin binding to actin. Consistent with this action, the Ca
2+ binding affinity of troponin has been reported to decrease with increasing blebbistatin and increase with an increase in sarcomere length [
18]. Monitoring [Ca
2+]
i in blebbistatin-treated myocytes, we show that stretch elicits a greater increase in [Ca
2+]
i compared to stretch in untreated controls (
Figure 3B,E). To dissect the contribution of X-ROS to the regulation of the global Ca
2+ transient, we treated myocytes with gp91ds-TAT (a peptide inhibitor of NOX2), or colchicine (to destabilize the microtubule network, see [
1]. The X-ROS signaling phenomena is the increased activation of RyR leading to increased Ca
2+ sparks induced by stretching the myocyte. Stretching the myocytes deforms the microtubular network that activates NOX2 to produce ROS. Previous studies with DCF fluorescence showed that ROS was increased during stretching [
6]. The DCF fluorescence measurements were not repeated here. Colchicine was used to reduce the microtubular network and hence the activation of NOX2. X-ROS suppression with either method significantly decreased peak of the global Ca
2+ transient with stretch, essentially unmasking the increase in Troponin C Ca
2+ affinity with stretch (
Figure 3C,E).
Informed by these new experimental results on the global transient, we conducted numerical experiments. In order to model the effects of blebbistatin, experimental data quantifying the effects of blebbistatin on the Ca
2+ affinity of troponin were considered. According to the observation by Farman et al. [
18], there was a decrease in calcium sensitivity with an increase in the blebbistatin concentration. However, using the approximately linear fit suggested in the paper, the length-dependent differences in the calcium binding affinity of troponin remained relatively constant between 0 and 1 µM blebbistatin, as seen by the difference between the two lines in
Figure 4A. On the other hand, fitting the EC
50 data for calcium sensitivity to the Hill equation (adaptive fit) gave a better least squares error than a linear fit for both the short and long sarcomere lengths, the result being that length-dependent differences disappear as the blebbistatin concentration increases (
Figure 4B). To assess which of these interpretations was more likely, we carried out two sets of simulations considering the data for the linear and adaptive cases. For the first set of simulations, we considered that the length-dependent change in the calcium binding affinity of troponin is the same in the presence or absence of blebbistatin (
Figure 4C, linear) and hence, when the myocyte is stretched, the
and
increases by 20% in both the presence of blebbistatin and the absence of blebbistatin (
Figure 5D, ctrl). For the second set of simulations, we considered that blebbistatin (at 3 µM concentration) would inhibit the length-dependent change in the calcium binding affinity of troponin (
Figure 4D, adaptive) and hence, when the myocyte is stretched, the
and
remain unchanged. The results of the second set of simulations (i.e., adaptive) resembled the experimental data (
Figure 3E, blebb) more closely than the results of the first set of simulations did (i.e., linear least sq.).
Using the adaptive fit described above, the simulation data were used to simulate the experiments displayed in
Figure 3. In the control simulations (
Figure 5A), the
rates for both the high and low affinity binding sites were increased by 20% when the myocyte was stretched to simulate the effects of length-dependent change in Ca
2+ affinity displayed by troponin [
18]. With this affinity increase, there was a small transient increase in [Ca
2+]
i (
Figure 5A). Removal of this increase in affinity to simulate the presence of blebbistatin (adaptive case) resulted in the expected almost doubling of the increase [Ca
2+]
i level during the stretching (
Figure 5B) over control stretching (
Figure 5A), similar to experimental data. Additionally, the block of X-ROS signaling to simulate the presence of gp91ds shows the buffering effect of troponin that increases during stretching (
Figure 5C). The peak [Ca
2+]
i during the stimulation every 2 s were averaged for rest, stretched and released conditions, and corresponding ΔF/F0 values were calculated, and normalized (
Figure 5D). These data sets show results similar to experiments (
Figure 3E). When stretched, the peak normalized ΔF/F0 increased by 2.2% in the control case whereas by 6% when there was no change in the Ca
2+ binding affinity of troponin (blebb). When no X-ROS was produced (gp91ds), the peak normalized ΔF/F0 decreased by 4.6%. The agreement of the experiment and simulation indicate the relative contributions of X-ROS signaling and myofilament calcium buffering by troponin to stretching-induced changes in [Ca
2+]
i transient amplitudes.
The concentration of Ca
2+ bound to troponin ([Ca
2+]
trpn) was determined in each condition (control, blebb and gp91ds), displayed in
Figure 6, using the same set of simulations as in
Figure 5. During the 0.5 Hz pacing protocol, the peak [Ca
2+]
trpn was 158 µM in the control condition, increased with myocyte stretch, and returned to its initial level when the cell was returned to resting length (
Figure 6A). Numerical experiments with blebbistatin revealed a significant drop in the peak [Ca
2+]
trpn at baseline and with stretch consistent with the reduced troponin Ca
2+ binding affinity (
Figure 6B). In contrast is our modeling of gp91ds, which inhibits stretch-dependent X-ROS independent of the stretch-dependent increase in [Ca
2+]
trpn (
Figure 6C).
4. Discussion
We conducted an integrated series of simulations and experiments to define the interplay between X-ROS signaling and the length-dependent changes in the Ca
2+ binding affinity of troponin. The initial model predictions suggested that with stretching, the transient increase in myoplasmic [Ca
2+] activated by X-ROS signaling would be reduced by the length-dependent increase in buffering by troponin due to the increase in Ca
2+ binding affinity. The model also suggested that in the contracting myocyte, this behavior would result in a rapid and transient decrease in [Ca
2+]
i due to length-dependent troponin sensitization followed by a small transient rise in [Ca
2+]
i due to X-ROS-dependent activation of the RyR. Upon relaxation, there would then be a small transient increase in myoplasmic [Ca
2+] due to the reversal of troponin sensitization. In fact, these predictions were consistent with the observation by Backx and ter Keurs, who reported a transient increase in myoplasmic Ca
2+ at the end of a twitch that they attributed to length-dependent changes in troponin dynamics [
20].
Experiments to dissect the relative contribution of X-ROS signaling from myofilament calcium buffering during stretch used blebbistatin to reduce the length-dependent effects on Ca
2+ binding by troponin. While recent results suggest that blebbistatin may reduce troponin Ca
2+ affinity independent of having an impact on length-dependent activation [
18], this conclusion was inconsistent with the experimental data presented (see
Figure 3). Our examination of these results leads us to suspect that the use of a linear fit to describe the data underlies this inconsistency. Support for this possibility comes from our use of an adaptive fit in which the reduction in troponin Ca
2+ binding affinity with increasing blebbistatin was accompanied by a reduction in and eventual elimination of length-dependent differences in binding affinity. In fact, the mean squared error of a linear vs. adaptive fit revealed the latter to be superior for the experimental data presented in
Figure 4. Furthermore, the adaptive fit enabled our model to faithfully reproduce the experimental result. Together, both the experimental results and model simulations suggest that blebbistatin acts in a concentration-dependent manner to progressively reduce, then eventually eliminate, the length dependence of Ca
2+ binding to troponin.
The new model offers insight into the interplay between X-ROS signaling and myofilament calcium buffering. The interaction between the regulation of stretch-dependent Ca
2+ spark behavior and cytosolic [Ca
2+] can be observed. During stretching, the increase in troponin Ca
2+ affinity—if unopposed—would lead to a transient reduction in myoplasmic [Ca
2+]. However, if there is a simultaneous rapid burst of X-ROS signaling that increases the sensitivity of RyR2 to activation by Ca
2+-induced Ca
2+ release (CICR), a rapid activation of Ca
2+ spark activity could occur. Given the importance of length-dependent myofilament Ca
2+ binding and activation in the regulation of cardiac contractility, the burst of X-ROS signaling with stretch appears to be an important mechanism that offsets the consequences of increased Ca
2+ affinity by troponin. In contrast, during the return of the myofilaments to resting length from stretching, the kinetics of the decrease in Ca
2+ binding to troponin and the decrease in Ca
2+ spark activity due to the decline of X-ROS signaling are critical to the time-course of [Ca
2+]
i. Despite the impact of the X-ROS burst rapidly waning, the acute reduction in the Ca
2+ buffering by troponin may transiently increase myoplasmic [Ca
2+]. If so, CICR would increase and there would be a brief delay in the return of Ca
2+ spark activity to the levels before stretching (
Figure 2D).
The interactions of X-ROS signaling, and myofilament calcium buffering was further interrogated by using blebbistatin to ablate length-dependent troponin sensitization of troponin and gp91ds to inhibit NOX2 and thus X-ROS signaling. Consistent with blebbistatin acting to inhibit the length-dependent increase in affinity of troponin for Ca2+, its effect in both experiments and model simulations was to increase stretch-dependent Ca2+ spark activation and hasten the return of Ca2+ spark activity upon a return to resting length. In the case of inhibiting X-ROS with gp91ds-TAT, both the experiment and model reveal a reduction in [Ca2+] with stretching which is consistent with a decrease in X-ROS-dependent RyR2 activation in the face of the increased troponin Ca2+ affinity during stretch. In contrast, is the impact of X-ROS inhibition during the return to resting length following stretching. Here, the model returns Ca2+ spark activity to the levels before stretching while the experimental system does not. We take this incongruency between experiment and model results as potential evidence of a target or effect of X-ROS signaling not yet included in the model.
Studies have suggested that in cardiac myocytes, there is an abrupt increase in force after stretching (rapid response) and a slower response that evolves over several minutes (slow force response). A potential mechanism for the slow force response has been suggested, including the activation of stretch-activated non-specific cation channels and paracrine/autocrine signaling. The slow force response has been shown to be helpful in normal function and detrimental in diseases such as heart failure [
21]. In the rapid response, when the heart muscle is stretched, there is an increase in force seen in the same action potential. Previous studies have suggested that this is due to a decrease in myofilament overlap and an increase in the Ca
2 sensitivity of the myofilaments (i.e., troponin) [
7]. Our previous studies have shown that the X-ROS mechanism can contribute to both the rapid response and the slow frequency response [
5]. The X-ROS mechanism causes stretching just prior to a contraction to result in the optimal augmentation of contraction. This response is attenuated upon subsequent beats but does contribute to be helpful in normal function. With oxidative stress, which is common in heart failure, the X-ROS mechanism is stronger and contributes to larger Ca
2+, which might be arrhythmogenic [
1,
5]. The inclusion of length-dependent changes in troponin Ca
2+ binding affinity lessens this response through increased buffering during stretch but will not abolish it completely. In other diseases such as Duchenne muscular dystrophy, X-ROS signaling has been suggested to play a role in triggering spontaneous Ca
2+ release, which can lead to arrhythmias [
1].
Another mechanism in which stretching can modify heart function is mechanoelectrical feedback. Mechanoelectrical feedback is thought to play a role in health and disease. In pacemaker cells, it has been suggested that the acute stretch cause by blood returning to the heart can lead to more rapid depolarization, increasing heart rate. In atria, stretching has been associated with sustained atrial fibrillation. In ventricles, stretch has been associated with increased arrhythmias [
22]. X-ROS signaling might play a role here as well. Some ion channels have been shown to alter function due to ROS. Calcium has also been shown to feed back on the action potential. While both of these mechanisms are possible, further study is needed to determine if there is any significant role.