Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis
Abstract
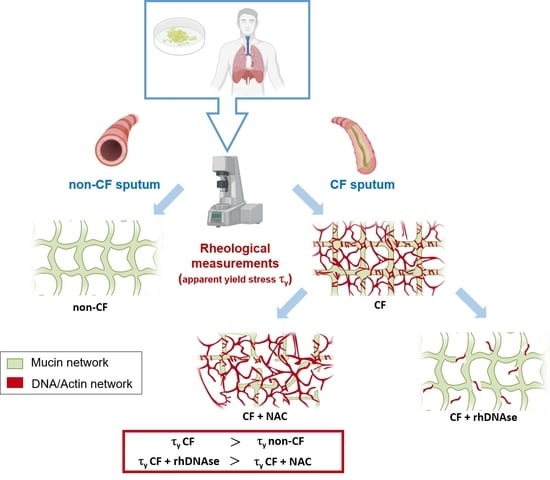
:1. Introduction
2. Materials and Methods
2.1. Sputum Collection
2.2. Patient Demographics
2.3. Rheological Experiments
2.4. Ex Vivo Treatment of Mucus
2.5. Statistical Analyses
3. Results
3.1. Preliminary Rheometrical Results
3.2. Linear and Non-Linear Rheological Characterization
3.3. Ex Vivo Sputum Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Dhooghe, B.; Noel, S.; Huaux, F.; Leal, T. Lung inflammation in cystic fibrosis: Pathogenesis and novel therapies. Clin. Biochem. 2014, 47, 539–546. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Elexacaftor/Ivacaftor/Tezacaftor: First Approval. Drugs 2019, 79, 2001–2007. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Munck, A.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; et al. Real-Life Safety and Effectiveness of Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 188–197. [Google Scholar] [CrossRef]
- Safirstein, J.; Grant, J.J.; Clausen, E.; Savant, D.; Dezube, R.; Hong, G. Biliary disease and cholecystectomy after initiation of elexacaftor/ivacaftor/tezacaftor in adults with cystic fibrosis. J. Cyst. Fibros. 2020, 20, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Baker, A.; Baker, E.; Boyd, A.C.; Cheng, S.H.; Coles, R.L.; Collie, D.D.S.; Davidson, H.; Davies, J.C.; Gill, D.R.; et al. The safety profile of a cationic lipid-mediated cystic fibrosis gene transfer agent following repeated monthly aerosol administration to sheep. Biomaterials 2013, 34, 10267–10277. [Google Scholar] [CrossRef] [Green Version]
- Duncan, G.; Jung, J.; Hanes, J.; Suk, J.S. The Mucus Barrier to Inhaled Gene Therapy. Mol. Ther. 2016, 24, 2043–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.H.; Litt, M.; Forsman, W.C. Rheological properties of mucus. Rheol. Acta 1969, 8, 438–448. [Google Scholar] [CrossRef]
- Puchelle, E.; Zahm, J.; Quemada, D. Rheological properties controlling mucociliary frequency and respiratory mucus transport. Biorheology 1987, 24, 557–563. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Zahm, J.; Pierrot, D.; Vaquez-Girod, S.; Puchelle, E. The role of mucus gel viscosity, spinnability, and adhesive properties in clearance by simulated cough. Biorheology 1989, 26, 737–745. [Google Scholar] [CrossRef]
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serisier, D.J.; Carroll, M.P.; Shute, J.K.; Young, S.A. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir. Res. 2009, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patarin, J.; Ghiringhelli, E.; Darsy, G.; Obamba, M.; Bochu, P.; Camara, B.; Quétant, S.; Cracowski, J.-L.; Cracowski, C.; Vincent, M.R.D.S. Rheological analysis of sputum from patients with chronic bronchial diseases. Sci. Rep. 2020, 10, 15685. [Google Scholar] [CrossRef]
- Ewoldt, R.; Hosoi, A.E.; McKinley, G. New measures for characterizing nonlinear viscoelasticity in large amplitude oscillatory shear. J. Rheol. 2008, 52, 1427–1458. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.T.; Tang, C.; Kang, L.; Voynow, J.A.; Rubin, B.K. Cystic Fibrosis Sputum Rheology Correlates With Both Acute and Longitudinal Changes in Lung Function. Chest 2018, 154, 370–377. [Google Scholar] [CrossRef]
- Møller, P.C.F.; Mewis, J.; Bonn, D. Yield stress and thixotropy: On the difficulty of measuring yield stresses in practice. Soft Matter 2006, 2, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Berret, J.-F.; Séréro, Y. Evidence of Shear-Induced Fluid Fracture in Telechelic Polymer Networks. Phys. Rev. Lett. 2001, 87, 048303. [Google Scholar] [CrossRef]
- Aubry, T.; Moan, M. Rheological behavior of a hydrophobically associating water soluble polymer. J. Rheol. 1994, 38, 1681–1692. [Google Scholar] [CrossRef]
- Bossard, F.; Aubry, T.; Gotzamanis, G.; Tsitsilianis, C. pH-Tunable rheological properties of a telechelic cationic polyelectrolyte reversible hydrogel. Soft Matter 2006, 2, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Shak, S. Aerosolized Recombinant Human DNase I for the Treatment of Cystic Fibrosis. Chest 1995, 107, 65S–70S. [Google Scholar] [CrossRef]
- Vukosavljevic, B.; Murgia, X.; Schwarzkopf, K.; Schaefer, U.F.; Lehr, C.-M.; Windbergs, M. Tracing molecular and structural changes upon mucolysis with N-acetyl cysteine in human airway mucus. Int. J. Pharm. 2017, 533, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Lethem, M.I.; James, S.L.; Marriott, C.; Burke, J.F. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur. Respir. J. 1990, 3, 19–23. [Google Scholar] [PubMed]
- Kirchner, K.K.; Wagener, J.S.; Khan, T.Z.; Copenhaver, S.C.; Accurso, F.J. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, O.W.; Yakubov, G.E.; Bonilla, M.R.; Deshmukh, O.; McGuckin, M.A.; Gidley, M.J. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+-mediated links, and hydrogen bonding. Sci. Rep. 2018, 8, 5802. [Google Scholar] [CrossRef] [Green Version]
- Kater, A.; Henke, M.O.; Rubin, B.K. The Role of DNA and Actin Polymers on the Polymer Structure and Rheology of Cystic Fibrosis Sputum and Depolymerization by Gelsolin or Thymosin Beta. Ann. N. Y. Acad. Sci. 2007, 1112, 140–153. [Google Scholar] [CrossRef]
- Vasconcellos, C.A.; Allen, P.G.; Wohl, M.E.; Drazen, J.M.; Janmey, P.A.; Stossel, T.P. Reduction in Viscosity of Cystic Fibrosis Sputum in Vitro by Gelsolin. Science 1994, 263, 969–971. [Google Scholar] [CrossRef]
- Mrsny, R.; Daugherty, A.; Short, S.; Widmer, R.; Siegel, M.; Keller, G.-A. Distribution of DNA and Alginate in Purulent Cystic Fibrosis Sputum: Implications to Pulmonary Targeting Strategies. J. Drug Target. 1996, 4, 233–243. [Google Scholar] [CrossRef]
- Sheils, C.A.; Käs, J.; Travassos, W.; Allen, P.G.; Janmey, P.A.; Wohl, M.E.; Stossel, T.P. Actin filaments mediate DNA fiber formation in chronic inflammatory airway disease. Am. J. Pathol. 1996, 148, 919–927. [Google Scholar]
- Körstgens, V.; Flemming, H.-C.; Wingender, J.; Borchard, W. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. J. Microbiol. Methods 2001, 46, 9–17. [Google Scholar] [CrossRef]
- Peterson, B.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; Van Winkelhoff, A.-J.; Neut, D.; Stoodley, P.; Van Der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Gloag, E.S.; German, G.K.; Stoodley, P.; Wozniak, D.J. Viscoelastic properties of Pseudomonas aeruginosa variant biofilms. Sci. Rep. 2018, 8, 9691. [Google Scholar] [CrossRef] [Green Version]
- Brandt, T.; Breitenstein, S.; Von Der Hardt, H.; Tummler, B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax 1995, 50, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Ratjen, F.; Paul, K.; van Koningsbruggen, S.; Breitenstein, S.; Rietschel, E.; Nikolaizik, W.; for the BEAT Study Group. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: Influence of treatment with dornase alpha. Pediatr. Pulmonol. 2004, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, R.; Laurent, V.; Roquefort, P.; Haute, T.; Ramel, S.; Le Gall, T.; Aubry, T.; Montier, T. Optimizations of In Vitro Mucus and Cell Culture Models to Better Predict In Vivo Gene Transfer in Pathological Lung Respiratory Airways: Cystic Fibrosis as an Example. Pharmaceutics 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol. Med. 2021, 27, 436–450. [Google Scholar] [CrossRef] [PubMed]




| Patient | Birth Year | Gender * | Mutation | FEV1 | Bacteria | Sputum Induction |
|---|---|---|---|---|---|---|
| 1 | 1988 | W | F508 del/F508 del | 55% | Achromobacter spp. | No |
| 2 | 2000 | M | F508 del/F508 del | 88% | MSSA ** Staphylococcus aureus | No |
| 3 | 1993 | M | 357 del C/357 del C | 30% | P. aeruginosa | No |
| 4 | 1961 | M | N1303K/1898 + 5 G > A | 46% | P. aeruginosa Escherichia coli | rhDNAse |
| 5 | 1978 | M | W 1282 X/R117H | 73% | MSSA P. aeruginosa | rhDNAse |
| 6 | 1985 | W | F508 del/G542X | 30% | MSSA P. aeruginosa Stenotrophomonas maltophilia | No |
| 7 | 1991 | W | F508 del/F508 del | 49% | MSSA P. aeruginosa | No |
| 8 | 1996 | W | F508 del/F508 del | 35% | Achromobacter spp. P. aeruginosa | rhDNAse |
| 9 | 1987 | W | F508 del/F508 del | 30% | MRSA *** P. aeruginosa | No |
| 10 | 1975 | W | F508 del/F508 del | 53% | Burkholderia cenocepacia | No |
| 11 | 1987 | W | F508 del/91 (G-R) | 66% | P. aeruginosa | rhDNAse |
| 12 | 1974 | M | F508 del/F508 del | 45% | P. aeruginosa | rhDNAse |
| 13 | 2006 | M | F508 del/F508 del | 93% | Haemophilus influenza SSA | rhDNAse |
| 14 | 1989 | W | F508 del/w882x | 31% | P. aeruginosa Candida albicans | No |
| 15 | 1979 | M | F508 del/F508 del | 30% | MSSA P. aeruginosa | rhDNAse |
| 16 | 1980 | M | F508 del/G411X | 53% | MSSA P. aeruginosa | No |
| 17 | 1985 | W | F508 del/F508 del | 23% | Aspergilus fumigatus P. aeruginosa | rhDNAse |
| 18 | 1982 | M | F508 del/F508 del | 35% | P. aeruginosa | No |
| 19 | 1985 | W | F508 del/F508 del | 70% | MSSA P. aeruginosa | No |
| 20 | 2014 | M | F508 del/4005 G > A | 114% | H. influenza MSSA | No |
| Patient | Pretreatment | Oscillatory Shear | Steady Shear | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 rad.s−1 | 10 rad.s−1 | ||||||||
| G′ (Pa) | G″ (Pa) | tanδ (G″/G′) | G′ (Pa) | G″ (Pa) | tanδ (G″/G′) | η (Pa.s) | τy (Pa) | ||
| 1 | No | 5.3 | 1.8 | 0.34 | 10.3 | 2.5 | 0.24 | 290 | 13 |
| 2 | No | 36 | 13 | 0.36 | 81 | 19 | 0.23 | 1400 | 48 |
| 3 | No | 2.8 | 1.3 | 0.46 | 6.7 | 1.8 | 0.27 | 150 | 10 |
| 4 | rhDNAse | 1.2 | 0.5 | 0.42 | 2.6 | 0.75 | 0.29 | 35 | 2 |
| 5 | rhDNAse | 3.1 | 0.9 | 0.29 | 6.5 | 1.7 | 0.26 | 185 | 14 |
| 6 | No | 3.3 | 1.2 | 0.36 | 6.9 | 1.8 | 0.26 | 160 | 19 |
| 7 | No | 5.4 | 2 | 0.37 | 12.2 | 3.1 | 0.25 | 270 | 14 |
| 8 | rhDNAse | 2.0 | 1 | 0.50 | 4.6 | 1.4 | 0.30 | 70 | 4 |
| 9 | No | 0.2 | 0.1 | 0.50 | 0.7 | 0.36 | 0.51 | 7 | 0.1 |
| 10 | No | 9.5 | 3.7 | 0.39 | 20 | 4.5 | 0.23 | 400 | 14 |
| 11 | rhDNAse | 2.3 | 1.1 | 0.48 | 6.2 | 2 | 0.32 | 60 | 2 |
| 12 | rhDNAse | 1.6 | 0.9 | 0.56 | 4.5 | 1.5 | 0.33 | 60 | 3 |
| 13 | rhDNAse | 2.4 | 0.8 | 0.33 | 5.2 | 1.3 | 0.25 | 120 | 4 |
| 14 | No | 3.0 | 1.2 | 0.40 | 6.7 | 1.7 | 0.25 | 120 | 10 |
| 15 | rhDNAse | 2.5 | 1.3 | 0.52 | 8.6 | 3.1 | 0.36 | 80 | 2 |
| 16 | No | 4.7 | 1.4 | 0.30 | 10.7 | 2.3 | 0.21 | 200 | 13 |
| 17 | rhDNAse | 2.0 | 1.2 | 0.60 | 9.7 | 3.4 | 0.35 | 120 | 0.3 |
| 18 | No | 44 | 12 | 0.27 | 93 | 18 | 0.19 | 12,000 | 16 |
| 19 | No | 1.6 | 0.8 | 0.50 | 3.7 | 0.9 | 0.24 | 250 | 7 |
| 20 | No | 0.8 | 0.4 | 0.50 | 2.3 | 0.8 | 0.35 | 36 | 3 |
| non-CF | No | 0.5 | 0.3 | 0.60 | 1.8 | 0.8 | 0.44 | 20 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, R.; Roquefort, P.; Ramel, S.; Laurent, V.; Haute, T.; Le Gall, T.; Aubry, T.; Montier, T. Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis. Cells 2021, 10, 3107. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113107
Ghanem R, Roquefort P, Ramel S, Laurent V, Haute T, Le Gall T, Aubry T, Montier T. Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis. Cells. 2021; 10(11):3107. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113107
Chicago/Turabian StyleGhanem, Rosy, Philippe Roquefort, Sophie Ramel, Véronique Laurent, Tanguy Haute, Tony Le Gall, Thierry Aubry, and Tristan Montier. 2021. "Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis" Cells 10, no. 11: 3107. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113107