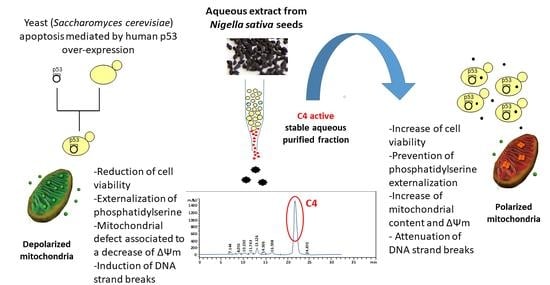
Whole and Purified Aqueous Extracts of Nigella sativa L. Seeds Attenuate Apoptosis and the Overproduction of Reactive Oxygen Species Triggered by p53 Over-Expression in the Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Media
2.2. Cell-Growth Kinetics
2.3. Extraction of Proteins and Western Blot
2.4. Nigella sativa L. Extract Preparation
2.5. Preparative High-Performance Liquid Chromatography (HPLC)
2.6. Preparative Fast-Performance Liquid Chromatography (FPLC)
2.7. Analytical High-Performance Liquid Chromatography (HPLC)
2.8. Precipitation with Cold Acetone/Ethanol
2.9. Radical Scavenging Activity
2.10. Survival Assay
2.11. Annexin V and Propidium Iodide (PI) Assay: Flow Cytometric Assay
2.12. Determination of Relative Cell Size and Granularity by Flow Cytometry
2.13. Flow Cytometric Quantification of the Relative Mitochondrial Content
2.14. Assessment of Intracellular Reactive Oxygen Species (ROS) Levels by Flow Cytometric Analysis Using H2-DCFDA
2.15. Flow Cytometric Assessment of Mitochondrial Membrane Potential after Staining with DiOC6(3)
2.16. Flow Cytometric Assessment of Mitochondrial Membrane Potential after Staining with JC-1
2.17. Statistical Analysis
3. Results
3.1. Anti-Apoptotic Activity of Aqueous Nigella sativa L. Extract
3.2. Effect of Aqueous Nigella Extract on p53 Expression
3.3. Purification of Anti-Apoptotic Molecules from Aqueous Nigella sativa L. Extract
3.4. Effect of the C4 Purified Fraction on Cell Viability
3.5. Antioxidant Activity of the C4 Fraction from Aqueous Nigella sativa L. Extract: An Evaluation by the DPPH Assay
3.6. Effect of the the C4 Fraction of Aqueous Nigella sativa L. Extract on the Apoptosis Markers
3.7. Effect of the C4 Fraction of Aqueous Nigella sativa L. Extract on Cell Size
3.8. Evaluation of the Mitochondrial Content
3.9. Effect of the C4 Fraction of Aqueous Nigella sativa L. Extract on the Reactive Oxygen Species (ROS) Production
3.10. Effect of the C4 Fraction of Aqueous Nigella sativa L. Extract on the Mitochondrial Membrane Potential (ΔΨm)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galluzzi, L.; Maiuri, M.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell death modalities: Classification and pathophysiological implications Morphological Characterization of Cell Death. Cell Death Differ. 2007, 14, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Jungwrth, H.; Lehmann, K.A.; Maldene, C.; Frohlich, K.U.; Wissing, S.; Buttner, S.; Fehr, M.; Sigrist, S.M.F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 16, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, M. Decision making by p53: Life, death and cancer. Cell Death Differ. 2003, 10, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Toufektchan, E.; Toledo, F. The Guardian of the Genome Revisited: p53 Downregulates Genes Required for Telomere Maintenance, DNA Repair, and Centromere Structure. Cancers 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duprez, L.; Wirawan, E.; Berghe, T.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef]
- Whelan, R.S.; Kaplinskiy, V.; Kitsis, R.N. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu. Rev. Physiol. 2010, 72, 19–44. [Google Scholar] [CrossRef]
- Gupta, S.; Kass, G.E.N.; Szegezdi, E.; Joseph, B. The mitochondrial death pathway: A promising therapeutic target in diseases. J. Cell. Mol. Med. 2009, 13, 1004–1033. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Morselli, E.; Kepp, O.; Vitale, I.; Rigoni, A.; Vacchelli, E.; Michaud, M.; Zischka, H.; Castedo, M.; Kroemer, G. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010, 31, 1–20. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell. Biol. 2008, 11, 621–632. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Ichim, G.; Green, D.R. Die another way--non-apoptotic mechanisms of cell death. J. Cell Sci. 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Lartigue, L.; Kushnareva, Y.; Seong, Y.; Lin, H.; Faustin, B.; Newmeyer, D.D. Caspase-independent Mitochondrial Cell Death Results from Loss of Respiration, Not Cytotoxic Protein Release. Mol. Biol. Cell 2009, 20, 4871–4884. [Google Scholar] [CrossRef] [Green Version]
- Olovnikov, I.A.; Kravchenko, J.E.; Chumakov, P.M. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Semin. Cancer Biol. 2009, 19, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Pawge, G.; Khatik, G.L. p53 regulated senescence mechanism and role of its modulators in age-related disorders. Biochem. Pharmacol. 2021, 190, 114651. [Google Scholar] [CrossRef]
- Pereira, C.; Bessa, C.; Soares, J.; Leo, M.; Saraiva, L. Contribution of yeast models to neurodegeneration research. J. Biomed. Biotechnol. 2012, 2012, 941232. [Google Scholar] [CrossRef] [Green Version]
- Nigro, J.M.; Sikorski, R.; Reed, S.I.; Vogelstein, B. Human p53 and CDC2Hs genes combine to inhibit the proliferation of Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 1357–1365. [Google Scholar] [CrossRef]
- Bureik, M.; Jungbluth, A.; Drescher, R.; Wagner, P. Human p53 restores DNA synthesis control in fission yeast. Biol. Chem. 1997, 378, 1361–1371. [Google Scholar] [CrossRef]
- Hadj Amor, I.Y.; Smaoui, K.; Chaabène, I.; Mabrouk, I.; Djemal, L.; Elleuch, H.; Allouche, M.; Mokdad-Gargouri, R.; Gargouri, A. Human p53 induces cell death and downregulates thioredoxin expression in Saccharomyces cerevisiae. FEMS Yeast Res. 2008, 8, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Aguilera, A.; Austriaco, N.; Ayscough, K.; Balzan, R.; Bar-Nun, S.; Barrientos, A.; Belenky, P.; et al. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Abdelmoula-Souissi, S.; Delahodde, A.; Bolotin-Fukuhara, M.; Gargouri, A.; Mokdad-Gargouri, R. Cellular localization of human p53 expressed in the yeast saccharomyces cerevisiae: Effect of NLSI deletion. Apoptosis 2011, 16, 746–756. [Google Scholar] [CrossRef]
- Bezine, M.; Namsi, A.; Sghaier, R.; Ben Khalifa, R.; Hamdouni, H.; Brahmi, F.; Badreddine, I.; Mihoubi, W.; Nury, T.; Vejux, A.; et al. The effect of oxysterols on nerve impulses. Biochimie 2018, 153, 46–51. [Google Scholar] [CrossRef]
- Nury, T.; Yammine, A.; Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Brahmi, F.; Samadi, M.; Rup-Jacques, S.; Vervandier-Fasseur, D.; Pais de Barros, J.P.; et al. Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing Res. Rev. 2021, 68, 101324. [Google Scholar] [CrossRef]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef]
- Thomas, B.J.; Rothstein, R. Elevated Recombination Rates in Transcriptionally Actve DNA. Cell 1989, 56, 619–630. [Google Scholar] [CrossRef]
- Cullin, C.; Pompon, D. Synthesis of functional mouse cytochromes P-450 P1 and chimeric P-450 P3-1 in the yeast Saccharomyces cerevisiae. Gene 1988, 65, 203–217. [Google Scholar] [CrossRef]
- Mokdad-Gargouri, R.; Belhadj, K.; Gargouri, A. Translational control of human p53 expression in yeast mediated by 5′-UTR-ORF structural interaction. Nucleic Acids Res. 2001, 29, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.S.; Gilani, A.H.; Khwaja, A.; Rashid, A.; Ashfaq, M.K. The in vitro effect of aqueous extract of nigella sativa seeds on nitric oxide production. Phyther. Res. 2003, 17, 921–924. [Google Scholar] [CrossRef]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Madeo, F.; Fröhlich, E.; Fröhlich, K.U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997, 139, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Volejníková, A.; Housková, J.; Sigler, K.; Pichová, A. Vital mitochondrial functions show profound changes during yeast culture ageing. FEMS Yeast Res. 2013, 13, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Madeo, F.; Fröhlich, E.; Ligr, M.; Grey, M.; Sigrist, S.J.; Wolf, D.H.; Fröhlich, K.U. Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 1999, 145, 757–767. [Google Scholar] [CrossRef]
- Chen, C.; Dickman, M.B. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 2004, 51, 1493–1507. [Google Scholar] [CrossRef]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Petros, A.M.; Olejniczak, E.T.; Fesik, S.W. Fesik Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 2004, 1644, 83–94. [Google Scholar] [CrossRef]
- Mannella, C.A. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim. Biophys. Acta 2006, 1762, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, L.; Hernández-García, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Koning, A.J.; Lum, P.Y.; Williams, J.M.; Wright, R. DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil. Cytoskelet. 1993, 25, 111–128. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Wendland, J. Apical localization of actin patches and vacuolar dynamics in Ashbya gossypii depend on the WASP homolog Wal1p. J. Cell Sci. 2004, 117, 4947–4958. [Google Scholar] [CrossRef] [Green Version]
- Cottet-Rousselle, C.; Ronot, X.; Leverve, X.; Mayol, J.F. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part A 2011, 79A, 405–425. [Google Scholar] [CrossRef]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef] [Green Version]
- Mbarek, L.A.; Ait Mouse, H.; Elabbadi, N.; Bensalah, M.; Gamouh, A.; Aboufatima, R.; Benharref, A.; Chait, A.; Kamal, M.; Dalal, A.; et al. Anti-tumor properties of blackseed (Nigella sativa L.) extracts. Braz. J. Med. Biol. Res. 2007, 40, 839–847. [Google Scholar] [CrossRef]
- Vayssière, J.L.; Petit, P.X.; Risler, Y.; Mignotte, B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl. Acad. Sci. USA 1994, 91, 11752–11756. [Google Scholar] [CrossRef] [Green Version]
- Reber, S.; Goehring, N. Intracellular Scaling Mechanisms. Cold Spring Harb. Perspect. Biol. 2015, 7, a019067. [Google Scholar] [CrossRef] [Green Version]
- Schmoller, K.M.; Skotheim, J.M. Special Issue: Quantitative Cell Biology The Biosynthetic Basis of Cell Size Control. Trends Cell Biol. 2015, 25, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Kitami, T.; Logan, D.J.; Negri, J.; Hasaka, T.; Tolliday, N.J.; Carpenter, A.E.; Spiegelman, B.M.; Mootha, V.K. A Chemical Screen Probing the Relationship between Mitochondrial Content and Cell Size. PLoS ONE 2012, 7, e33755. [Google Scholar] [CrossRef] [Green Version]
- Posakony, J.W.; England, J.M.; Attardi, G. Mitochondrial growth and division during the cell cycle in HeLa cells. J. Cell Biol. 1977, 74, 468–491. [Google Scholar] [CrossRef] [Green Version]
- Rafelski, S.M.; Viana, M.P.; Zhang, Y.; Chan, Y.-H.M.; Thorn, K.S.; Yam, P.; Fung, J.C.; Li, H.; Costa, L.D.F.; Marshall, W.F. Mitochondrial Network Size Scaling in Budding Yeast. Science 2012, 338, 822–824. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Energy Conversion: Mitochondria and Chloroplasts. In Molecular Biology of the Cell; U.S. National Library of Medicine: Bethesda, MD, USA, 2002. [Google Scholar]
- Renner, K.; Amberger, A.; Konwalinka, G.; Kofler, R.; Gnaiger, E. Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim. Biophys. Acta 2003, 1642, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Yamaki, M.; Umehara, T.; Chimura, T.; Horikoshi, M. Cell death with predominant apoptotic features in Saccharomyces cerevisiae mediated by deletion of the histone chaperone ASF1/CIA1. Genes Cells 2001, 6, 1043–1054. [Google Scholar] [CrossRef]
- Du, L.; Yu, Y.; Chen, J.; Liu, Y.; Xia, Y.; Chen, Q.; Liu, X. Arsenic induces caspase- and mitochondria-mediated apoptosis in Saccharomyces cerevisiae. FEMS Yeast Res. 2007, 7, 860–865. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Gajate, C.; Yu, L.; Fang, Y.; Mollinedo, F. Mitochondrial-derived ROS in edelfosine-induced apoptosis in yeasts and tumor cells. Acta Pharmacol. Sin. 2007, 28, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Sapienza, K.; Bannister, W.; Balzan, R. Mitochondrial involvement in aspirin-induced apoptosis in yeast. Microbiology 2008, 154, 2740–2747. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Büttner, S.; Kroemer, G.; Madeo, F. The mitochondrial pathway in yeast apoptosis. Apoptosis 2007, 74, 527–534. [Google Scholar] [CrossRef]
- Pereira, C.; Silva, R.D.; Saraiva, L.; Johansson, B.; Sousa, M.J.; Côrte-Real, M. Mitochondria-dependent apoptosis in yeast. Biochim. Biophys. Acta -Mol. Cell Res. 2008, 1783, 1286–1302. [Google Scholar] [CrossRef] [Green Version]
- Breitenbach, M.; Laun, P.; Gimona, M. The actin cytoskeleton, RAS–cAMP signaling and mitochondrial ROS in yeast apoptosis. Trends Cell Biol. 2005, 15, 637–639. [Google Scholar] [CrossRef]
- Burhans, W.C.; Weinberger, M.; Marchetti, M.A.; Ramachandran, L.; D’Urso, G.; Huberman, J.A. Apoptosis-like yeast cell death in response to DNA damage and replication defects. Mutat. Res. 2003, 532, 227–243. [Google Scholar] [CrossRef]
- Munoz, A.J.; Wanichthanarak, K.; Meza, E.; Petranovic, D. Systems biology of yeast cell death. FEMS Yeast Res. 2012, 12, 249–265. [Google Scholar] [CrossRef]
- Yang, K.-M.; Lee, N.-R.; Woo, J.-M.; Choi, W.; Zimmermann, M.; Blank, L.M.; Park, J.-B. Ethanol reduces mitochondrial membrane integrity and thereby impacts carbon metabolism of Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Hwang, B.; Hwang, J.-S.; Lee, J.; Kim, J.-K.; Kim, S.R.; Kim, Y.; Lee, D.G. Induction of yeast apoptosis by an antimicrobial peptide, Papiliocin. Biochem. Biophys. Res. Commun. 2011, 408, 89–93. [Google Scholar] [CrossRef]
- Ludovico, P.; Rodrigues, F.; Almeida, A.; Silva, M.T.; Barrientos, A.; Côrte-Real, M. Cytochrome c Release and Mitochondria Involvement in Programmed Cell Death Induced by Acetic Acid in Saccharomyces cerevisiae. Mol. Biol. Cell 2002, 13, 2598–2606. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Chandel, N.S.; Schumacker, P.T.; Thompson, C.B. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 1999, 3, 159–167. [Google Scholar] [CrossRef]
- Malakar, D.; Dey, A.; Basu, A.; Ghosh, A.K. Antiapoptotic role of S-adenosyl-l-methionine against hydrochloric acid induced cell death in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2008, 1780, 937–947. [Google Scholar] [CrossRef]
- Severin, F.F.; Hyman, A.A. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 2002, 12, R233–R235. [Google Scholar] [CrossRef] [Green Version]
- Pozniakovsky, A.I.; Knorre, D.A.; Markova, O.V.; Hyman, A.A.; Skulachev, V.P.; Severin, F.F. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J. Cell Biol. 2005, 168, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Susin, S.A.; Zamzami, N.; Kroemer, G. Mitochondria as regulators of apoptosis: Doubt no more. Biochim. Biophys. Acta 1998, 1366, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Chandel, N.S.; Williamson, E.K.; Schumacker, P.T.; Thompson, C.B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 1997, 91, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.; Pilcher, K.; Blachly-Dyson, E.; Basso, E.; Jockel, J.; Bassik, M.C.; Korsmeyer, S.J.; Forte, M. Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-X(L). Mol. Cell. Biol. 2000, 20, 3125–3136. [Google Scholar] [CrossRef] [Green Version]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Li, P.F.; Dietz, R.; von Harsdorf, R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 1999, 18, 6027–6036. [Google Scholar] [CrossRef]
- Pfanner, N.; Craig, E.A.; Meijer, M. The Protein Import Machinery of the Mitochondrial Inner Membrane. Trends Biochem. Sci. 1994, 9, 368–372. [Google Scholar]
- Wong, J.R.; Bo Chen, L. Recent Advances in the Study of Mitochondria in Living Cells. Adv. Mol. Cell Biol. 1988, 2, 263–290. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihoubi, W.; Sahli, E.; Rezgui, F.; Dabebi, N.; Sayehi, R.; Hassairi, H.; Masmoudi-Fourati, N.; Walha, K.; ben Khadhra, K.; Baklouti, M.; et al. Whole and Purified Aqueous Extracts of Nigella sativa L. Seeds Attenuate Apoptosis and the Overproduction of Reactive Oxygen Species Triggered by p53 Over-Expression in the Yeast Saccharomyces cerevisiae. Cells 2022, 11, 869. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11050869
Mihoubi W, Sahli E, Rezgui F, Dabebi N, Sayehi R, Hassairi H, Masmoudi-Fourati N, Walha K, ben Khadhra K, Baklouti M, et al. Whole and Purified Aqueous Extracts of Nigella sativa L. Seeds Attenuate Apoptosis and the Overproduction of Reactive Oxygen Species Triggered by p53 Over-Expression in the Yeast Saccharomyces cerevisiae. Cells. 2022; 11(5):869. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11050869
Chicago/Turabian StyleMihoubi, Wafa, Emna Sahli, Fatma Rezgui, Najeh Dabebi, Rabiaa Sayehi, Hajer Hassairi, Najla Masmoudi-Fourati, Kamel Walha, Khalifa ben Khadhra, Mohamed Baklouti, and et al. 2022. "Whole and Purified Aqueous Extracts of Nigella sativa L. Seeds Attenuate Apoptosis and the Overproduction of Reactive Oxygen Species Triggered by p53 Over-Expression in the Yeast Saccharomyces cerevisiae" Cells 11, no. 5: 869. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11050869