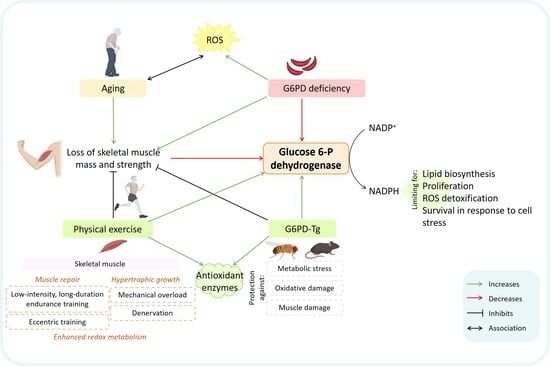
Glucose 6-P Dehydrogenase—An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise
Abstract
:1. The Pentose Phosphate Pathway and the Regulation of G6PD
2. Loss of Function Models for G6PD
3. G6PD and Cell Growth
4. G6PD in the Regeneration of Skeletal Muscle after Damage
5. Positive Regulators of G6PD Activity in Skeletal Muscle—Role of Exercise
| Positive Regulators | Negative Regulators |
|---|---|
| Acetylation [130] | 5′ adenosine monophosphate-activated protein kinase (AMPK) [131] |
| G6PD activator AG1 [132] | Aldosterone [133] |
| AKT [134] | Angiotensin II [120] |
| ATM serine/threonine kinase (ATM) [135] | Arachidonic acid [136] |
| Benfotiamine (vitamin B1 analog) [110,137] | Cyclic adenosine monophosphate (cAMP) [138] |
| Proto-oncogene tyrosine-protein kinase Src (c-Src) [25] | cAMP-dependent protein kinase A [138] |
| cGMP-dependent protein kinase G [139] | cAMP response element modulator (CREM) [133] |
| Cyclin D3-CDK6 [140] | Dehydroepiandrosterone (DHEA) [141] |
| Epidermal growth factor (EGF) [142] | miR-122 and miR-1 [143] |
| Estrogens [110] | p38 mitogen-activated protein kinase [136] |
| Exercise [32] | p53 [144] |
| Glycosylation [145] | Phosphatase and tensin homolog (PTEN) [146] |
| Growth hormone [110] | TP53 [144] |
| Hepatocyte growth factor (HGF) [147] | Tumor necrosis factor-α (TNFα) [68] |
| Heat shock protein 27 (Hsp27) [148] | |
| Hypoxia inducible factor (HIF) [149] | |
| Inhibitor of DNA binding 1 (ID1) [150] | |
| Insulin [151] | |
| Mammalian target of rapamycin (mTOR) [152] | |
| Nuclear-factor-E2-related factor (Nrf2) [153] | |
| Ribosomal protein S6 kinase beta-1 (p70S6K) [110] | |
| Serine/threonine-protein kinase PAK 4 (PAK4) [154] | |
| Protein disulfide isomerase family A, member 3 pseudogene (PDIA3P) [155] | |
| Phosphatidylinositol-3-kinase (PI-3K) [134] | |
| Phospholipase C [110] | |
| Phospholipase C-γ [156] | |
| Platelet-derived growth factor (PDGF) [156] | |
| Polo-like kinase 1 (PLK-1) [157] | |
| Ras-GTPase [68] | |
| S6 kinase [158] | |
| Snail [159] | |
| Sterol-responsive element bindingprotein (SREBP) 1 [68] | |
| Stobadine [160] | |
| TAp73 [161] | |
| Testosterone [110] | |
| Transforming growth factor beta 1 (TGF-β1) [162] | |
| TP53-induced glycolysis and apoptosis regulator (TIGAR) [163] | |
| Vascular endothelial cell growth factor (VEGF) [25] | |
| Vitamin D [164] | |
| Vitamin E [160] |
Author Contributions
Funding
Conflicts of Interest
References
- Couri, D.; Racker, E. The oxidative pentose phosphate cycle. V. Complete oxidation of glucose 6-phosphate in a reconstructed system of the oxidative pentose phosphate cycle. Arch. Biochem. Biophys. 1959, 83, 195–205. [Google Scholar] [CrossRef]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [PubMed]
- Dickens, F.; Williamson, D.H. Pentose phosphate isomerase and epimerase from animal tissues. Biochem. J. 1956, 64, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sulek, K. Nobel prize in 1931 for Otto Warburg for discovery of the respiratory enzyme. Wiad. Lek. 1968, 21, 329. [Google Scholar]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Baquer, N.Z.; Hothersall, J.S.; McLean, P. Function and regulation of the pentose phosphate pathway in brain. Curr. Top. Cell. Regul. 1988, 29, 265–289. [Google Scholar]
- Barcia-Vieitez, R.; Ramos-Martínez, J.I. The regulation of the oxidative phase of the pentose phosphate pathway: New answers to old problems. IUBMB Life 2014, 66, 775–779. [Google Scholar] [CrossRef]
- Ghergurovich, J.M.; García-Cañaveras, J.C.; Wang, J.; Schmidt, E.; Zhang, Z.; TeSlaa, T.; Patel, H.; Chen, L.; Britt, E.C.; Piqueras-Nebot, M.; et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Nobrega-Pereira, S. NADPH: New oxygen for the ROS theory of aging. Oncotarget 2016, 7, 50814–50815. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr.; Stryer, L.; Held, A.T.; Maxam, G.T.; Seidler, L.T.; Hacker, B.R.T.; Jarosch, B.T. Biochemistry, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Cho, E.S.; Cha, Y.H.; Kim, H.S.; Kim, N.H.; Yook, J.I. The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomol. Ther. 2018, 26, 29–38. [Google Scholar] [CrossRef]
- Eggleston, L.V.; Krebs, H.A. Regulation of the pentose phosphate cycle. Biochem. J. 1974, 138, 425–435. [Google Scholar] [CrossRef]
- Salati, L.M.; Amir-Ahmady, B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu. Rev. Nutr. 2001, 21, 121–140. [Google Scholar] [CrossRef]
- Jiang, A.; Guo, H.; Jiang, X.; Tao, J.; Wu, W.; Liu, H. G6PD Deficiency is Crucial for Insulin Signaling Activation in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 7425. [Google Scholar] [CrossRef]
- Horton, J.D. Sterol regulatory element-binding proteins: Transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 2002, 30, 1091–1095. [Google Scholar] [CrossRef]
- Salati, L.M.; Szeszel-Fedorowicz, W.; Tao, H.; Gibson, M.A.; Amir-Ahmady, B.; Stabile, L.P.; Hodge, D.L. Nutritional regulation of mRNA processing. J. Nutr. 2004, 134, 2437S–2443S. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wang, S.Q. Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem. Pharmacol. 2007, 73, 1358–1366. [Google Scholar] [CrossRef]
- Holten, D.; Procsal, D.; Chang, H.L. Regulation of pentose phosphate pathway dehydrogenases by NADP+/NADPH ratios. Biochem. Biophys. Res. Commun. 1976, 68, 436–441. [Google Scholar] [CrossRef]
- Garcia, A.A.; Mathews, I.I.; Horikoshi, N.; Matsui, T.; Kaur, M.; Wakatsuki, S.; Mochly-Rosen, D. Stabilization of glucose-6-phosphate dehydrogenase oligomers enhances catalytic activity and stability of clinical variants. J. Biol. Chem. 2022, 298, 101610. [Google Scholar] [CrossRef]
- Veech, R.L.; Eggleston, L.V.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1969, 115, 609–619. [Google Scholar] [CrossRef]
- Pan, S.; World, C.J.; Kovacs, C.J.; Berk, B.C. Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 895–901. [Google Scholar] [CrossRef]
- Marks, P.A.; Gross, R.T. Erythrocyte glucose-6-phosphate dehydrogenase deficiency: Evidence of differences between Negroes and Caucasians with respect to this genetically determined trait. J. Clin. Investig. 1959, 38, 2253–2262. [Google Scholar] [CrossRef]
- Frank, J.E. Diagnosis and management of G6PD deficiency. Am. Fam. Physician 2005, 72, 1277–1282. [Google Scholar]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Ruwende, C.; Khoo, S.C.; Snow, R.W.; Yates, S.N.; Kwiatkowski, D.; Gupta, S.; Warn, P.; Allsopp, C.E.; Gilbert, S.C.; Peschu, N. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995, 376, 246–249. [Google Scholar] [CrossRef]
- Cappadoro, M.; Giribaldi, G.; O’Brien, E.; Turrini, F.; Mannu, F.; Ulliers, D.; Simula, G.; Luzzatto, L.; Arese, P. Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood 1998, 92, 2527–2534. [Google Scholar] [CrossRef]
- Kletzien, R.F.; Harris, P.K.; Foellmi, L.A. Glucose-6-phosphate dehydrogenase: A “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994, 8, 174–181. [Google Scholar] [CrossRef]
- Arc-Chagnaud, C.; Salvador-Pascual, A.; Garcia-Dominguez, E.; Olaso-Gonzalez, G.; Correas, A.G.; Serna, E.; Brioche, T.; Chopard, A.; Fernandez-Marcos, P.J.; Serrano, M.; et al. Glucose 6-P dehydrogenase delays the onset of frailty by protecting against muscle damage. J. Cachexia Sarcopenia Muscle 2021, 12, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Arese, P. Favism and Glucose-6-Phosphate Dehydrogenase Deficiency. N. Engl. J. Med. 2018, 378, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Hecker, P.A.; Lionetti, V.; Ribeiro, R.F.; Rastogi, S.; Brown, B.H.; O’Connell, K.A.; Cox, J.W.; Shekar, K.C.; Gamble, D.M.; Sabbah, H.N.; et al. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ. Heart Fail. 2013, 6, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalev, O.; Wollner, A.; Menczel, J. Diabetic ketoacidosis does not precipitate haemolysis in patients with the Mediterranean variant of glucose-6-phosphate dehydrogenase deficiency. Br. Med. J. 1984, 288, 179–180. [Google Scholar] [CrossRef]
- Lee, D.H.; Warkentin, T.E.; Neame, P.B.; Ali, M.A. Acute hemolytic anemia precipitated by myocardial infarction and pericardial tamponade in G6PD deficiency. Am. J. Hematol. 1996, 51, 174–175. [Google Scholar] [CrossRef]
- Battistuzzi, G.; D’Urso, M.; Toniolo, D.; Persico, G.M.; Luzzatto, L. Tissue-specific levels of human glucose-6-phosphate dehydrogenase correlate with methylation of specific sites at the 3′ end of the gene. Proc. Natl. Acad. Sci. USA 1985, 82, 1465–1469. [Google Scholar] [CrossRef]
- Nagashio, R.; Oikawa, S.; Yanagita, K.; Hagiuda, D.; Kuchitsu, Y.; Igawa, S.; Naoki, K.; Satoh, Y.; Ichinoe, M.; Murakumo, Y.; et al. Prognostic significance of G6PD expression and localization in lung adenocarcinoma. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 38–46. [Google Scholar] [CrossRef]
- Vives Corrons, J.L.; Feliu, E.; Pujades, M.A.; Cardellach, F.; Rozman, C.; Carreras, A.; Jou, J.M.; Vallespí, M.T.; Zuazu, F.J. Severe-glucose-6-phosphate dehydrogenase (G6PD) deficiency associated with chronic hemolytic anemia, granulocyte dysfunction, and increased susceptibility to infections: Description of a new molecular variant (G6PD Barcelona). Blood 1982, 59, 428–434. [Google Scholar] [CrossRef]
- Longo, L.; Vanegas, O.C.; Patel, M.; Rosti, V.; Li, H.; Waka, J.; Merghoub, T.; Pandolfi, P.P.; Notaro, R.; Manova, K.; et al. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 2002, 21, 4229–4239. [Google Scholar] [CrossRef]
- Nicol, C.J.; Zielenski, J.; Tsui, L.C.; Wells, P.G. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 2000, 14, 111–127. [Google Scholar] [CrossRef]
- Pretsch, W.; Charles, D.J.; Merkle, S. X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem. Genet. 1988, 26, 89–103. [Google Scholar] [CrossRef]
- Sanders, S.; Smith, D.P.; Thomas, G.A.; Williams, E.D. A glucose-6-phosphate dehydrogenase (G6PD) splice site consensus sequence mutation associated with G6PD enzyme deficiency. Mutat. Res. 1997, 374, 79–87. [Google Scholar] [CrossRef]
- Spolarics, Z.; Condon, M.R.; Siddiqi, M.; Machiedo, G.W.; Deitch, E.A. Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2118–H2126. [Google Scholar] [CrossRef]
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—Beyond the realm of red cell biology. Free Radic. Res. 2014, 48, 1028–1048. [Google Scholar] [CrossRef]
- Wan, G.H.; Tsai, S.C.; Chiu, D.T. Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine 2002, 19, 191–195. [Google Scholar] [CrossRef]
- Lai, Y.K.; Lai, N.M.; Lee, S.W. Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: A systematic review and meta-analysis. Ann. Hematol. 2017, 96, 839–845. [Google Scholar] [CrossRef]
- Heymann, A.D.; Cohen, Y.; Chodick, G. Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes Care 2012, 35, e58. [Google Scholar] [CrossRef]
- Cappai, G.; Songini, M.; Doria, A.; Cavallerano, J.D.; Lorenzi, M. Increased prevalence of proliferative retinopathy in patients with type 1 diabetes who are deficient in glucose-6-phosphate dehydrogenase. Diabetologia 2011, 54, 1539–1542. [Google Scholar] [CrossRef]
- Niazi, G.A. Glucose-6-phosphate dehydrogenase deficiency and diabetes mellitus. Int. J. Hematol. 1991, 54, 295–298. [Google Scholar]
- Carette, C.; Dubois-Laforgue, D.; Gautier, J.F.; Timsit, J. Diabetes mellitus and glucose-6-phosphate dehydrogenase deficiency: From one crisis to another. Diabetes Metab. 2011, 37, 79–82. [Google Scholar] [CrossRef]
- Díaz-Flores, M.; Ibáñez-Hernández, M.A.; Galván, R.E.; Gutiérrez, M.; Durán-Reyes, G.; Medina-Navarro, R.; Pascoe-Lira, D.; Ortega-Camarillo, C.; Vilar-Rojas, C.; Cruz, M.; et al. Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci. 2006, 78, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liew, C.W.; Handy, D.E.; Zhang, Y.; Leopold, J.A.; Hu, J.; Guo, L.; Kulkarni, R.N.; Loscalzo, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J. 2010, 24, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Hu, J.; Stillman, I.E.; Leopold, J.A.; Handy, D.E.; Loscalzo, J.; Stanton, R.C. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J. 2010, 24, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.T.; Walsh, M.E.; Van Remmen, H. Mouse Models of Oxidative Stress Indicate a Role for Modulating Healthy Aging. J. Clin. Exp. Pathol. 2012, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Brandes, R.P.; Weissmann, N.; Schroder, K. NADPH oxidases in cardiovascular disease. Free. Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef]
- Penna, C.; Mancardi, D.; Rastaldo, R.; Pagliaro, P. Cardioprotection: A radical view Free radicals in pre and postconditioning. Biochim. Biophys. Acta 2009, 1787, 781–793. [Google Scholar] [CrossRef]
- Carretero, A.; Gomez-Cabrera, M.C.; Rios-Navarro, C.; Salvador-Pascual, A.; Bodi, V.; Viña, J. Early reductive stress and late onset overexpression of antioxidant enzymes in experimental myocardial infarction. Free Radic. Res. 2020, 54, 173–184. [Google Scholar] [CrossRef]
- Hecker, P.A.; Leopold, J.A.; Gupte, S.A.; Recchia, F.A.; Stanley, W.C. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H491–H500. [Google Scholar] [CrossRef]
- Matsui, R.; Xu, S.; Maitland, K.A.; Mastroianni, R.; Leopold, J.A.; Handy, D.E.; Loscalzo, J.; Cohen, R.A. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E(−/−) mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 910–916. [Google Scholar] [CrossRef]
- Cocco, P.; Todde, P.; Fornera, S.; Manca, M.B.; Manca, P.; Sias, A.R. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood 1998, 91, 706–709. [Google Scholar] [CrossRef]
- Jeng, W.; Loniewska, M.M.; Wells, P.G. Brain glucose-6-phosphate dehydrogenase protects against endogenous oxidative DNA damage and neurodegeneration in aged mice. ACS Chem. Neurosci. 2013, 4, 1123–1132. [Google Scholar] [CrossRef]
- Loniewska, M.M.; Gupta, A.; Bhatia, S.; MacKay-Clackett, I.; Jia, Z.; Wells, P.G. DNA damage and synaptic and behavioural disorders in glucose-6-phosphate dehydrogenase-deficient mice. Redox Biol. 2020, 28, 101332. [Google Scholar] [CrossRef]
- Tian, W.N.; Braunstein, L.D.; Pang, J.; Stuhlmeier, K.M.; Xi, Q.C.; Tian, X.; Stanton, R.C. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J. Biol. Chem. 1998, 273, 10609–10617. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.N.; Braunstein, L.D.; Apse, K.; Pang, J.; Rose, M.; Tian, X.; Stanton, R.C. Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am. J. Physiol. 1999, 276, C1121–C1131. [Google Scholar] [CrossRef]
- Pandolfi, P.P.; Sonati, F.; Rivi, R.; Mason, P.; Grosveld, F.; Luzzatto, L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995, 14, 5209–5215. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- Farquhar, J.K.; Scott, W.N.; Coe, F.L. Hexose monophosphate shunt activity in compensatory renal hypertrophy. Proc. Soc. Exp. Biol. Med. 1968, 129, 809–812. [Google Scholar] [CrossRef]
- Schaffer, W.T. Effects of growth hormone on lipogenic enzyme activities in cultured rat hepatocytes. Am. J. Physiol. 1985, 248, E719–E725. [Google Scholar] [CrossRef]
- Sulis, E. G.-6-PD deficiency and cancer. Lancet 1972, 1, 1185. [Google Scholar] [CrossRef]
- Beaconsfield, P. Local metabolic response to physio-pathological demands: The pentose phosphate pathway. Experientia 1963, 19, 437–438. [Google Scholar] [CrossRef]
- Beatty, C.H.; Peterson, R.D.; Basinger, G.M.; Bocek, R.M. Major metabolic pathways for carbohydrate metabolism of voluntary skeletal muscle. Am. J. Physiol. 1966, 210, 404–410. [Google Scholar] [CrossRef]
- Wagner, K.R.; Kauffman, F.C.; Max, S.R. The pentose phosphate pathway in regenerating skeletal muscle. Biochem. J. 1978, 170, 17–22. [Google Scholar] [CrossRef]
- Rifenberick, D.H.; Koski, C.L.; Max, S.R. Metabolic studies of skeletal muscle regeneration. Exp. Neurol. 1974, 45, 527–540. [Google Scholar] [CrossRef]
- Smith, B. Histochemical changes in muscle necrosis and regeneration. J. Pathol. Bacteriol. 1965, 89, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.H. Metabolic activity during the degenerative and early regenerative stages of minced skeletal muscle. Anat. Rec. 1973, 176, 185–203. [Google Scholar] [CrossRef]
- Beaconsfield, P.; Carpi, A. Localization of an infectious lesion and glucose metabolism via the pentose phosphate pathway. Nature 1964, 201, 825–827. [Google Scholar] [CrossRef]
- Boveris, A.; Erecinska, M.; Wagner, M. Reduction kinetics of cytochromes b. Biochim. Biophys. Acta 1972, 256, 223–242. [Google Scholar] [CrossRef]
- Beaconsfield, P.; Reading, H.W. Pathways of glucose metabolism and nucleic acid synthesis. Nature 1964, 202, 464–466. [Google Scholar] [CrossRef]
- Susheela, A.K.; Hudgson, P.; Walton, J.N. Murine muscular dystrophy. Some histochemical and biochemical observations. J. Neurol. Sci. 1968, 7, 437–463. [Google Scholar] [CrossRef]
- Carlson, B.M. Regeneration of the rat gastrocnemius muscle from sibling and non-sibling muscle fragments. Am. J. Anat. 1970, 128, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Relationship between the tissue and epimorphic regeneration of muscles. Am. Zool. 1970, 10, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lee-Young, R.S.; Hoffman, N.J.; Murphy, K.T.; Henstridge, D.C.; Samocha-Bonet, D.; Siebel, A.L.; Iliades, P.; Zivanovic, B.; Hong, Y.H.; Colgan, T.D.; et al. Glucose-6-phosphate dehydrogenase contributes to the regulation of glucose uptake in skeletal muscle. Mol. Metab. 2016, 5, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Kimmick, G.; Owen, J. Rhabdomyolysis and hemolysis associated with sickle cell trait and glucose-6-phosphate dehydrogenase deficiency. South Med. J. 1996, 89, 1097–1098. [Google Scholar] [CrossRef]
- Meijer, A.E.; Elias, E.A. The inhibitory effect of actinomycin D and cycloheximide on the increase in activity of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in experimentally induced diseased skeletal muscles. Histochem. J. 1984, 16, 971–982. [Google Scholar] [CrossRef]
- Ninfali, P.; Baronciani, L.; Bardoni, A.; Bresolin, N. Muscle expression of glucose-6-phosphate dehydrogenase deficiency in different variants. Clin. Genet. 1995, 48, 232–237. [Google Scholar] [CrossRef]
- Legan, S.K.; Rebrin, I.; Mockett, R.J.; Radyuk, S.N.; Klichko, V.I.; Sohal, R.S.; Orr, W.C. Overexpression of glucose-6-phosphate dehydrogenase extends the life span of Drosophila melanogaster. J. Biol. Chem. 2008, 283, 32492–32499. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Reclos, G.J.; Parthimos, T.; Parthimos, N.; Gavriilidis, A.; Tsakiris, S. L-cysteine supplementation protects the erythrocyte glucose-6-phosphate dehydrogenase activity from reduction induced by forced training. Clin. Biochem. 2006, 39, 1002–1006. [Google Scholar] [CrossRef]
- Tsakiris, S.; Reclos, G.J.; Parthimos, T.; Tsakiris, T.; Parthimos, N.; Schulpis, K.H. α-Tocopherol supplementation restores the reduction of erythrocyte glucose-6-phosphate dehydrogenase activity induced by forced training. Pharmacol. Res. 2006, 54, 373–379. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Tsironi, M.; Skenderi, K.; Lazaropoulou, C.; Parthimos, N.; Reclos, G.; Goussetis, E.; Tsakiris, S.; Papassotiriou, I. Dramatic reduction of erythrocyte glucose-6-phosphate dehydrogenase activity in athletes participating in the ultradistance foot race “Spartathlon”. Scand. J. Clin. Lab. Investig. 2008, 68, 228–232. [Google Scholar] [CrossRef]
- Tsakiris, S.; Parthimos, T.; Reclos, G.J.; Parthimos, N.; Tsakiris, T.; Schulpis, K.H. Significant reduction of erythrocyte glucose-6-phosphate dehydrogenase activity in soccer-players during play. Evidence for catecholamine mediated enzyme inhibition. Clin. Chem. Lab. Med. 2009, 47, 621–624. [Google Scholar] [CrossRef]
- Boström, S.; Fahlén, M.; Hjalmarson, A.; Johansson, R. Activities of rat muscle enzymes after acute exercise. Acta Physiol. Scand. 1974, 90, 544–554. [Google Scholar] [CrossRef]
- Schwane, J.A.; Armstrong, R.B. Effect of training on skeletal muscle injury from downhill running in rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 969–975. [Google Scholar] [CrossRef]
- Farenia, R.; Lesmana, R.; Uchida, K.; Iwasaki, T.; Koibuchi, N.; Shimokawa, N. Changes in biomarker levels and myofiber constitution in rat soleus muscle at different exercise intensities. Mol. Cell. Biochem. 2019, 458, 79–87. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Ogilvie, R.W.; Schwane, J.A. Eccentric exercise-induced injury to rat skeletal muscle. J. Appl. Physiol. 1983, 54, 80–93. [Google Scholar] [CrossRef]
- Boström, S.; Fahlen, M.; Hjalmarsson, A.; Johansson, R.G. Muscle enzyme activities after repeated ischemia. Int. J. Biochem. 1974, 5, 359–363. [Google Scholar] [CrossRef]
- Gudbjarnason, S.; Braasch, W.; Cowan, C.; Bing, R.J. Metabolism of infarcted heart muscle during tissue repair. Am. J. Cardiol. 1968, 22, 360–369. [Google Scholar] [CrossRef]
- Valentino, T.; Figueiredo, V.C.; Mobley, C.B.; McCarthy, J.J.; Vechetti, I.J. Evidence of myomiR regulation of the pentose phosphate pathway during mechanical load-induced hypertrophy. Physiol. Rep. 2021, 9, e15137. [Google Scholar] [CrossRef]
- Turner, L.V.; Manchester, K.L. Glucose and glycogen metabolism in hypertrophied denervated rat hemidiaphragm. Biochem. J. 1970, 117, 33P. [Google Scholar] [CrossRef]
- Weyrauch, L.A.; McMillin, S.L.; Witczak, C.A. Insulin Resistance Does Not Impair Mechanical Overload-Stimulated Glucose Uptake, but Does Alter the Metabolic Fate of Glucose in Mouse Muscle. Int. J. Mol. Sci. 2020, 21, 4715. [Google Scholar] [CrossRef]
- Izumiya, Y.; Hopkins, T.; Morris, C.; Sato, K.; Zeng, L.; Viereck, J.; Hamilton, J.A.; Ouchi, N.; LeBrasseur, N.K.; Walsh, K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008, 7, 159–172. [Google Scholar] [CrossRef]
- Wu, C.L.; Satomi, Y.; Walsh, K. RNA-seq and metabolomic analyses of Akt1-mediated muscle growth reveals regulation of regenerative pathways and changes in the muscle secretome. BMC Genom. 2017, 18, 181. [Google Scholar] [CrossRef]
- Tullson, P.; Armstrong, R.B. Muscle hexose monophosphate shunt activity following exercise. Experientia 1981, 37, 1311–1312. [Google Scholar] [CrossRef]
- Novak, M.L.; Billich, W.; Smith, S.M.; Sukhija, K.B.; McLoughlin, T.J.; Hornberger, T.A.; Koh, T.J. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1132–R1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, A.; Dong, C.; Li, B.; Zhang, Z.; Chen, Y.; Ning, C.; Wu, W.; Liu, H. MicroRNA-206 regulates cell proliferation by targeting G6PD in skeletal muscle. FASEB J. 2019, 33, 14083–14094. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Kawata, K.; Kunida, K.; Hatano, A.; Yugi, K.; Wada, T.; Fujii, M.; Sano, T.; Ito, Y.; Furuichi, Y.; et al. Trans-omic Analysis Reveals ROS-Dependent Pentose Phosphate Pathway Activation after High-Frequency Electrical Stimulation in C2C12 Myotubes. iScience 2020, 23, 101558. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.J.; Patel, R.M.; McClintock, T.S.; Dupont-Versteegden, E.E.; Peterson, C.A.; McCarthy, J.J. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol. Biol. Cell 2016, 27, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, E.L.; Hikim, A.P.; Shen, R.; Sinha, I.; Sinha-Hikim, I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010, 151, 628–638. [Google Scholar] [CrossRef]
- Brioche, T.; Kireev, R.A.; Cuesta, S.; Gratas-Delamarche, A.; Tresguerres, J.A.; Gomez-Cabrera, M.C.; Viña, J. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: Improvement of protein balance and of antioxidant defenses. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 1186–1198. [Google Scholar] [CrossRef]
- Laguens, R.P.; Gómez Dumm, C.L. Deoxyribonucleic acid synthesis in the heart mitochondria after acute and exhaustive exercise. Experientia 1968, 24, 163–164. [Google Scholar] [CrossRef]
- Place, N.; Ivarsson, N.; Venckunas, T.; Neyroud, D.; Brazaitis, M.; Cheng, A.J.; Ochala, J.; Kamandulis, S.; Girard, S.; Volungevicius, G.; et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. USA 2015, 112, 15492–15497. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Melikoglu, M.A.; Kaldirimci, M.; Katkat, D.; Sen, I.; Kaplan, I.; Senel, K. The effect of regular long term training on antioxidant enzymatic activities. J. Sports Med. Phys. Fit. 2008, 48, 388–390. [Google Scholar]
- Spodaryk, K.; Szyguła, Z.; Dabrowski, Z.; Miszta, H. The activity of erythrocyte enzymes in rats subjected to running exercises. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 533–537. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Borrás, C.; Pallardó, F.V.; Sastre, J.; Ji, L.L.; Viña, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Brioche, T.; Pagano, A.F.; Py, G.; Chopard, A. Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention. Mol. Aspects Med. 2016, 50, 56–87. [Google Scholar] [CrossRef]
- Herscovich, S.; Gershon, D. Effects of aging and physical training on the neuromuscular junction of the mouse. Gerontology 1987, 33, 7–13. [Google Scholar] [CrossRef]
- Griffiths, M.A.; Baker, D.H.; Novakofski, J.E.; Ji, L.L. Effects of exercise training on diet-induced lipogenic enzymes and body composition in rats. J. Am Coll. Nutr. 1993, 12, 155–161. [Google Scholar] [CrossRef]
- Pereira, B.; Costa Rosa, L.F.; Safi, D.A.; Medeiros, M.H.; Curi, R.; Bechara, E.J. Superoxide dismutase, catalase, and glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol. Behav. 1994, 56, 1095–1099. [Google Scholar] [CrossRef]
- Borges-Silva, C.N.; Fonseca-Alaniz, M.H.; Alonso-Vale, M.I.; Takada, J.; Andreotti, S.; Peres, S.B.; Cipolla-Neto, J.; Pithon-Curi, T.C.; Lima, F.B. Reduced lipolysis and increased lipogenesis in adipose tissue from pinealectomized rats adapted to training. J. Pineal. Res. 2005, 39, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Bresolin, N. Muscle glucose 6-phosphate dehydrogenase (G6PD) deficiency and oxidant stress during physical exercise. Cell Biochem. Funct. 1995, 13, 297–298. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The Effects of Acute Low-Volume HIIT and Aerobic Exercise on Leukocyte Count and Redox Status. J. Sports Sci. Med. 2018, 17, 501–508. [Google Scholar]
- Georgakouli, K.; Fatouros, I.G.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Deli, C.K.; Jamurtas, A.Z. Exercise in Glucose-6-Phosphate Dehydrogenase Deficiency: Harmful or Harmless? A Narrative Review. Oxid. Med. Cell. Longev. 2019, 2019, 8060193. [Google Scholar] [CrossRef] [Green Version]
- Jamurtas, A.Z.; Fatouros, I.G.; Koukosias, N.; Manthou, E.; Tofas, T.; Yfanti, C.; Nikolaidis, M.G.; Koutedakis, Y. Effect of exercise on oxidative stress in individuals with glucose-6-phosphate dehydrogenase deficiency. In Vivo 2006, 20, 875–880. [Google Scholar]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Sakellariou, G.K.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. Comparison between glucose-6-phosphate dehydrogenase-deficient and normal individuals after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 1113–1121. [Google Scholar] [CrossRef]
- Makarona, K.; Caputo, V.S.; Costa, J.R.; Liu, B.; O’Connor, D.; Iskander, D.; Roper, D.; Robertson, L.; Bhatnagar, N.; Terpos, E.; et al. Transcriptional and epigenetic basis for restoration of G6PD enzymatic activity in human G6PD-deficient cells. Blood 2014, 124, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, Z.; Yao, J.; Tan, R.; Zhu, Y.; Li, Z.; Guo, Q.; Wei, L. Regulation of AMPK-related glycolipid metabolism imbalances redox homeostasis and inhibits anchorage independent growth in human breast cancer cells. Redox Biol. 2018, 17, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Mruk, K.; Rahighi, S.; Raub, A.G.; Chen, C.H.; Dorn, L.E.; Horikoshi, N.; Wakatsuki, S.; Chen, J.K.; Mochly-Rosen, D. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat. Commun. 2018, 9, 4045. [Google Scholar] [CrossRef]
- Leopold, J.A.; Dam, A.; Maron, B.A.; Scribner, A.W.; Liao, R.; Handy, D.E.; Stanton, R.C.; Pitt, B.; Loscalzo, J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat. Med. 2007, 13, 189–197. [Google Scholar] [CrossRef]
- Wagle, A.; Jivraj, S.; Garlock, G.L.; Stapleton, S.R. Insulin regulation of glucose-6-phosphate dehydrogenase gene expression is rapamycin-sensitive and requires phosphatidylinositol 3-kinase. J. Biol. Chem. 1998, 273, 14968–14974. [Google Scholar] [CrossRef]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef]
- Talukdar, I.; Szeszel-Fedorowicz, W.; Salati, L.M. Arachidonic acid inhibits the insulin induction of glucose-6-phosphate dehydrogenase via p38 MAP kinase. J. Biol. Chem. 2005, 280, 40660–40667. [Google Scholar] [CrossRef]
- Katare, R.; Caporali, A.; Emanueli, C.; Madeddu, P. Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J. Mol. Cell. Cardiol. 2010, 49, 625–638. [Google Scholar] [CrossRef]
- Zhang, Z.; Apse, K.; Pang, J.; Stanton, R.C. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J. Biol. Chem. 2000, 275, 40042–40047. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Kandhi, S.; Kelly, M.; Neo, B.H.; Wolin, M.S. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L383–L391. [Google Scholar] [CrossRef]
- Wang, H.; Nicolay, B.N.; Chick, J.M.; Gao, X.; Geng, Y.; Ren, H.; Gao, H.; Yang, G.; Williams, J.A.; Suski, J.M.; et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 2017, 546, 426–430. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Pashko, L.L. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res. Rev. 2004, 3, 171–187. [Google Scholar] [CrossRef]
- Tsao, M.S.; Earp, H.S.; Grisham, J.W. The effects of epidermal growth factor and the state of confluence on enzymatic activities of cultured rat liver epithelial cells. J. Cell. Physiol. 1986, 126, 167–173. [Google Scholar] [CrossRef]
- Köberle, V.; Kronenberger, B.; Pleli, T.; Trojan, J.; Imelmann, E.; Peveling-Oberhag, J.; Welker, M.W.; Elhendawy, M.; Zeuzem, S.; Piiper, A.; et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur. J. Cancer 2013, 49, 3442–3449. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Duan, X.; Mao, W.; Li, X.; Li, Z.; Li, Q.; Zheng, Z.; Xu, H.; Chen, M.; Wang, P.G.; et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 2015, 6, 8468. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Song, R.; Song, H.; Zheng, T.; Wang, J.; Liang, Y.; Qi, S.; Lu, Z.; Song, X.; Jiang, H.; et al. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut 2014, 63, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Aird, K.M.; Worth, A.J.; Snyder, N.W.; Lee, J.V.; Sivanand, S.; Liu, Q.; Blair, I.A.; Wellen, K.E.; Zhang, R. ATM couples replication stress and metabolic reprogramming during cellular senescence. Cell Rep. 2015, 11, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Préville, X.; Salvemini, F.; Giraud, S.; Chaufour, S.; Paul, C.; Stepien, G.; Ursini, M.V.; Arrigo, A.P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999, 247, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard, W.A.; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 2012, 337, 975–980. [Google Scholar] [CrossRef]
- Yin, X.; Tang, B.; Li, J.H.; Wang, Y.; Zhang, L.; Xie, X.Y.; Zhang, B.H.; Qiu, S.J.; Wu, W.Z.; Ren, Z.G. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J. Exp. Clin. Cancer Res. 2017, 36, 166. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshimoto, K.; Aoyama, K.; Ichihara, A. Hormonal regulations of glucose-6-phosphate dehydrogenase and lipogenesis in primary cultures of rat hepatocytes. J. Biochem. 1982, 91, 681–693. [Google Scholar] [CrossRef]
- Tsouko, E.; Khan, A.S.; White, M.A.; Han, J.J.; Shi, Y.; Merchant, F.A.; Sharpe, M.A.; Xin, L.; Frigo, D.E. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis 2014, 3, e103. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Shao, Y.; Xiao, J.; Zhu, G.; Li, F. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017, 8, e2820. [Google Scholar] [CrossRef]
- Yang, X.; Ye, H.; He, M.; Zhou, X.; Sun, N.; Guo, W.; Lin, X.; Huang, H.; Lin, Y.; Yao, R.; et al. LncRNA PDIA3P interacts with c-Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem. Biophys. Res. Commun. 2018, 498, 207–213. [Google Scholar] [CrossRef]
- Tian, W.N.; Pignatare, J.N.; Stanton, R.C. Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J. Biol. Chem. 1994, 269, 14798–14805. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Huang, D.; Li, Y.; Yang, D.; Li, T.; Li, F.; Sun, L.; Wei, H.; He, K.; et al. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat. Commun. 2017, 8, 1506. [Google Scholar] [CrossRef]
- Thakur, A.; Rahman, K.W.; Wu, J.; Bollig, A.; Biliran, H.; Lin, X.; Nassar, H.; Grignon, D.J.; Sarkar, F.H.; Liao, J.D. Aberrant expression of X-linked genes RbAp46, Rsk4, and Cldn2 in breast cancer. Mol. Cancer Res. 2007, 5, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Cha, Y.H.; Lee, J.; Lee, S.H.; Yang, J.H.; Yun, J.S.; Cho, E.S.; Zhang, X.; Nam, M.; Kim, N.; et al. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat. Commun. 2017, 8, 14374. [Google Scholar] [CrossRef]
- Ulusu, N.N.; Sahilli, M.; Avci, A.; Canbolat, O.; Ozansoy, G.; Ari, N.; Bali, M.; Stefek, M.; Stolc, S.; Gajdosik, A.; et al. Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: Effects of stobadine and vitamin E. Neurochem. Res. 2003, 28, 815–823. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, F.; Ruan, S.; Hu, M.; Hu, Y.; Fang, Z.; Mei, L.; Gong, C. The TGFβ1-FOXM1-HMGA1-TGFβ1 positive feedback loop increases the cisplatin resistance of non-small cell lung cancer by inducing G6PD expression. Am. J. Transl. Res. 2019, 11, 6860–6876. [Google Scholar]
- Wang, J.; Duan, Z.; Nugent, Z.; Zou, J.X.; Borowsky, A.D.; Zhang, Y.; Tepper, C.G.; Li, J.J.; Fiehn, O.; Xu, J.; et al. Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes. Cancer Lett. 2016, 378, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Sardar, S.; Chakraborty, A.; Chatterjee, M. Comparative effectiveness of vitamin D3 and dietary vitamin E on peroxidation of lipids and enzymes of the hepatic antioxidant system in Sprague—Dawley rats. Int. J. Vitam. Nutr. Res. 1996, 66, 39–45. [Google Scholar]



| Class | Mutation Severity | % of Normal G6PD Function |
|---|---|---|
| Class I | Severe deficiency associated with chronic non-spherocytic hemolytic anemia | <1 |
| Class II | Residual activity associated with acute hemolytic anemia | 1–10 |
| Class III | Mild | 10–60 |
| Class IV | Normal activity | 60–150 |
| Class V | More than normal activity | >150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, E.; Carretero, A.; Viña-Almunia, A.; Domenech-Fernandez, J.; Olaso-Gonzalez, G.; Viña, J.; Gomez-Cabrera, M.C. Glucose 6-P Dehydrogenase—An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise. Cells 2022, 11, 3041. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11193041
García-Domínguez E, Carretero A, Viña-Almunia A, Domenech-Fernandez J, Olaso-Gonzalez G, Viña J, Gomez-Cabrera MC. Glucose 6-P Dehydrogenase—An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise. Cells. 2022; 11(19):3041. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11193041
Chicago/Turabian StyleGarcía-Domínguez, Esther, Aitor Carretero, Aurora Viña-Almunia, Julio Domenech-Fernandez, Gloria Olaso-Gonzalez, Jose Viña, and Mari Carmen Gomez-Cabrera. 2022. "Glucose 6-P Dehydrogenase—An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise" Cells 11, no. 19: 3041. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11193041