Cyclin B Export to the Cytoplasm via the Nup62 Subcomplex and Subsequent Rapid Nuclear Import Are Required for the Initiation of Drosophila Male Meiosis
Abstract
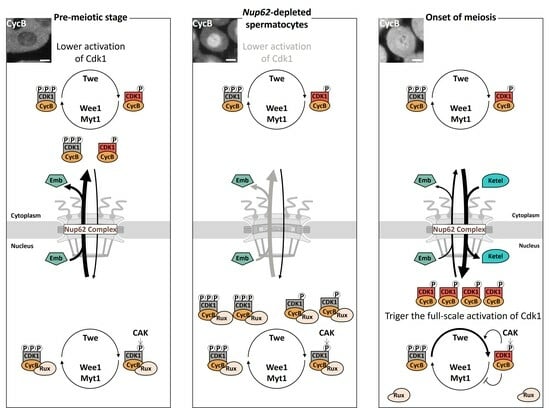
:1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks
2.2. Transformation
2.3. Preparation of Post-Meiotic Spermatids
2.4. Immunofluorescence
2.5. Live-Cell Imaging of Primary Spermatocytes
2.6. In Situ Proximity Ligation Assay (PLA)
2.7. Statistical Analysis
3. Results
3.1. CycB Exhibits Continuous Nuclear Import and Export via the Nup62 Subcomplex during the Growth Phase of Drosophila Spermatocytes before Meiosis
3.2. Overexpression of Normal CycB, but Not CycB Harboring a Nuclear Localization Signal (NLS), Rescued the Inhibition of Meiotic Initiation in Nup62-Depleted Spermatocytes
3.3. Cdk1 Showed Reduced Activation during the Growth Phase and Full-Scale Activation Immediately after Rapid Re-Entry of CycB in the Nucleus upon Meiosis Onset
3.4. CentrosomeSeparation in the Cytoplasm Prior to Full-Scale Cdk1 Activation Depended on Cdk1, Which Was Not Observed in Nup62-Silenced Spermatocytes
3.5. Cdk1 Was Closely Associated with CAK in the Nucleus during the Growth Phase in Both Normal and Nup62-Silenced Spermatocytes before Meiosis
3.6. The Association of Cdk1 with its Activator Phosphatase, Twine, in the Nucleus and Cytoplasm before Meiosis Did Not Change in Nup62-Silenced Cells
3.7. Silencing of the CKI Roughex (Rux), but Not Z600, Rescued the Nup62-Silencing-Induced Inhibition of Meiotic Initiation
3.8. Depletion of Importin β Inhibited Rapid Nuclear Re-Entry of CycB and Initiation of Meiosis but Did Not Affect Centrosome Separation before Meiosis
4. Discussion
4.1. Loss of Cytoplasmic CycB and Concomitantly Reduced Initial Cdk1 Activity Inhibit Meiosis in Nup62-Silenced Spermatocytes When Nuclear Export Is Disrupted
4.2. Cdk1 Activation May Be Suppressed in the Nuclei of Spermatocytes by Roughex until the Onset of Meiosis
4.3. Rapid Re-Entry of Cdk1–CycB to the Nucleus May Play an Important Role in the Full-Scale Activation of Cdk1 with Initial Activity and Meiotic Initiation
4.4. Stepwise Activation of Cdk1 Is Associated with Nuclear–Cytoplasmic Shuttling of CycB Mediated by the Nup62 Subcomplex, Exportin, and Importin β
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, D.O. The Cell Cycle: Principles of Control (Primers in Biology), 1st ed.; New Science Press: London, UK, 2007; p. 297. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan., D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Liu, F.; Stanton, J.J.; Wu, Z.; Piwnica-Worms, H. The Human Myt1 Kinase Preferentially Phosphorylates Cdc2 on Threonine 14 and Localizes to the Endoplasmic Reticulum and Golgi Complex. Mol. Cell. Biol. 1997, 17, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Coulonval, K.; Kooken, H.; Roger, P.P. Coupling of T161 and T14 phosphorylations protects cyclin B–CDK1 from premature activation. Mol. Biol. Cell 2011, 22, 3971–3985. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, J.O.; Varadarajan, R.; Mukherjee, O.; Stuart, D.T.; Sprenger, F.; Srayko, M.; Campbell, S.D. Dual Phosphorylation of Cdk1 Coordinates Cell Proliferation with Key Developmental Processes in Drosophila. Genetics 2014, 196, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Lolli, G.; Johnson, L.N. CAK-Cyclin-dependent Activating Kinase: A key kinase in cell cycle control and a target for drugs? Cell Cycle 2005, 4, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Dulić, V.; Stein, G.H.; Far, D.F.; Reed, S.I. Nuclear accumulation of p21Cip1 at the onset of mitosis: A role at the G2/M-phase transition. Mol. Cell. Biol. 1998, 18, 546–557. [Google Scholar] [CrossRef]
- Dash, B.C.; El-Deiry, W.S. Phosphorylation of P21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol. Cell. Biol. 2005, 25, 3364–3387. [Google Scholar] [CrossRef]
- Russo, A.A.; Jeffrey, P.D.; Pavletich, N.P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat. Struct. Biol. 1996, 3, 696–700. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Lindqvist, A.; Rodríguez-Bravo, V.; Medema, R.H. The decision to enter mitosis: Feedback and redundancy in the mitotic entry network. J. Cell Biol. 2009, 185, 193–202. [Google Scholar] [CrossRef]
- Qian, J.; Winkler, C.; Bollen, M. 4D-networking by mitotic phosphatases. Curr. Opin. Cell Biol. 2013, 25, 697–703. [Google Scholar] [CrossRef]
- Hégarat, N.; Rata, S.; Hochegger, H. Bistability of mitotic entry and exit switches during open mitosis in mammalian cells. BioEssays 2016, 38, 627–643. [Google Scholar] [CrossRef]
- Hiraoka, D.; Hosoda, E.; Chiba, K.; Kishimoto, T. SGK phosphorylates Cdc25 and Myt1 to trigger cyclin B–Cdk1 activation at the meiotic G2/M transition. J. Cell Biol. 2019, 218, 3597–3611. [Google Scholar] [CrossRef]
- Santos, S.D.M.; Wollman, R.; Meyer, T.; Ferrell, J.E. Spatial Positive Feedback at the Onset of Mitosis. Cell 2012, 149, 1500–1513. [Google Scholar] [CrossRef]
- Maryu, G.; Yang, Q. Nuclear-cytoplasmic compartmentalization of cyclin B1-Cdk1 promotes robust timing of mitotic events. Cell Rep. 2022, 41, 111870. [Google Scholar] [CrossRef]
- Crncec, A.; Hochegger, H. Triggering mitosis. FEBS Lett. 2019, 593, 2868–2888. [Google Scholar] [CrossRef] [PubMed]
- Pines, J.; Hunter, T. Cyclin-dependent kinases: A new cell cycle motif? Trends Cell Biol. 1991, 1, 117–121. [Google Scholar] [CrossRef]
- Hagting, A.; Karlsson, C.; Clute, P.; Jackman, M.; Pines, J. MPF localization is controlled by nuclear export. EMBO J. 1998, 17, 4127–4138. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.A.; Donoghue, D.J. Cyclin B1 and CDK1: Nuclear localization and upstream regulators. Prog. Cell Cycle Res. 2003, 5, 335–347. [Google Scholar] [PubMed]
- Gavet, O.; Pines, J. Progressive Activation of CyclinB1-Cdk1 Coordinates Entry to Mitosis. Dev. Cell 2010, 18, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.E.; Weaver, J.; Jones, K.T. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development 2010, 137, 1297–1304. [Google Scholar] [CrossRef]
- Inoue, Y.H.; Miyauchi, C.; Ogata, T.; Kitazawa, D. Dynamic alteration of cellular component of male meiosis in Drosophila. In Meiosis-Molecular Mechanisms and Cytogenetic Diversity; Swan, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 953–979. [Google Scholar]
- Sigrist, S.; Ried, G.; Lehner, C.F. Dmcdc2 kinase is required for both meiotic divisions during Drosophila spermatogenesis and is activated by the Twine/Cdc25 phosphatase. Mech. Dev. 1995, 53, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Courtot, C.; Fankhauser, C.; Simanis, V.; Lehner, C.F. The Drosophila Cdc25 homolog Twine is required for meiosis. Development 1992, 116, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Alphey, L.; Jimenez, J.; White-Cooper, H.; Dawson, I.; Nurse, P.; Glover, D.M. twine, a cdc25 homolog that functions in the male and female germline of drosophila. Cell 1992, 69, 977–988. [Google Scholar] [CrossRef] [PubMed]
- White-Cooper, H.; Alphey, L.; Glover, D.M. The Cdc25 homologue Twine is required for only some aspects of the entry into meiosis in Drosophila. J. Cell Sci. 1993, 106, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- White-Cooper, H.; Schäfer, M.A.; Alphey, L.S.; Fuller, M.T. Transcriptional and post-transcriptional control mechanisms coordinate the onset of spermatid differentiation with meiosis I in Drosophila. Development 1998, 125, 125–134. [Google Scholar] [CrossRef]
- Baker, C.C.; Gim, B.S.; Fuller, M.T. Cell type-specific translational repression of Cyclin B during meiosis in males. Development 2015, 142, 3394–3402. [Google Scholar] [CrossRef]
- Cenci, G.; Bonaccorsi, S.; Pisano, C.; Verni, F.; Gatti, M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 1994, 107, 3521–3534. [Google Scholar] [CrossRef]
- Azuma, M.; Ogata, T.; Yamazoe, K.; Tanaka, Y.; Inoue, Y.H. Heat shock cognate 70 genes contribute to Drosophila spermatocyte growth progression possibly through the insulin signaling pathway. Dev. Growth Differ. 2021, 63, 231–248. [Google Scholar] [CrossRef]
- Beck, M.; Hurt, E. The nuclear pore complex: Understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 2017, 18, 73–89. [Google Scholar] [CrossRef]
- Wing, C.E.; Fung, H.Y.J.; Chook, Y.M. Karyopherin-mediated nucleocytoplasmic transport. Nat. Rev. Mol. Cell Biol. 2022, 23, 307–328. [Google Scholar] [CrossRef]
- Kimura, M.; Imamoto, N. Biological significance of the importin-β family-dependent nucleocytoplasmic transport pathways. Traffic 2014, 15, 727–748. [Google Scholar] [CrossRef]
- Hayashi, D.; Tanabe, K.; Katsube, H.; Inoue, Y.H. B-Type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol. Open 2016, 5, 1011–1021. [Google Scholar] [CrossRef]
- Okazaki, R.; Yamazoe, K.; Inoue, Y.H. Nuclear Export of Cyclin B Mediated by the Nup62 Complex Is Required for Meiotic Initiation in Drosophila Males. Cells 2020, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gujar, M.R.; Deng, Q.; Chia, S.Y.; Li, S.; Tan, P.; Sung, W.-K.; Wang, H. Histone lysine methyltransferase pr-Set7/SETD8 promotes neural stem cell reactivation. EMBO Rep. 2021, 22, e50994. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.-Q.; Zhou, R.; Czech, B.; Liu, L.-P.; Holderbaum, L.; Yang-Zhou, D.; Shim, H.-S.; Tao, R.; Handler, D.; Karpowicz, P.; et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 2011, 8, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.; Chhangani, D.; Wren, M.C.; Smith, C.L.; Lee, J.H.; Li, X.; Puttinger, C.; Tsai, C.-W.; Fortin, G.; Morderer, D.; et al. Nuclear import receptors are recruited by FG-nucleoporins to rescue hallmarks of TDP-43 proteinopathy. Mol. Neurodegener. 2022, 17, 80. [Google Scholar] [CrossRef]
- Huang, J.; Raff, J.W. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 1999, 18, 2184–2195. [Google Scholar] [CrossRef]
- Schertel, C.; Huang, D.; Björklund, M.; Bischof, J.; Yin, D.; Li, R.; Wu, Y.; Zeng, R.; Wu, J.; Taipale, J.; et al. Systematic Screening of a Drosophila ORF Library In Vivo Uncovers Wnt/Wg Pathway Components. Dev. Cell 2013, 25, 207–219. [Google Scholar] [CrossRef]
- Liu, B.; Gregor, I.; Müller, H.-A.; Großhans, J. Fluorescence fluctuation analysis reveals PpV dependent Cdc25 protein dynamics in living embryos. PLoS Genet. 2020, 16, e1008735. [Google Scholar] [CrossRef]
- Oka, S.; Hirai, J.; Yasukawa, T.; Nakahara, Y.; Inoue, Y.H. A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Dienemann, A.; Sprenger, F. Requirements of Cyclin A for Mitosis Are Independent of Its Subcellular Localization. Curr. Biol. 2004, 14, 1117–1123. [Google Scholar] [CrossRef]
- Whitfield, W.G.; Gonzalez, C.; Maldonado-Codina, G.; Glover, D.M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990, 9, 2563–2572. [Google Scholar] [CrossRef]
- Novak, Z.A.; Conduit, P.T.; Wainman, A.; Raff, J.W. Asterless Licenses Daughter Centrioles to Duplicate for the First Time in Drosophila Embryos. Curr. Biol. 2014, 24, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Di Talia, S.; She, R.; Blythe, S.A.; Lu, X.; Zhang, Q.F.; Wieschaus, E.F. Posttranslational Control of Cdc25 Degradation Terminates Drosophila’s Early Cell-Cycle Program. Curr. Biol. 2013, 23, 127–132. [Google Scholar] [CrossRef]
- Tanabe, K.; Awane, R.; Shoda, T.; Yamazoe, K.; Inoue, Y.H. Mutations in mxc Tumor-Suppressor Gene Induce Chromosome Instability in Drosophila Male Meiosis. Cell Struct. Funct. 2019, 44, 121–135. [Google Scholar] [CrossRef]
- Foley, E.; O’Farrell, P.H.; Sprenger, F. Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin–Cdk complexes. Curr. Biol. 1999, 9, 1392–1402. [Google Scholar] [CrossRef]
- Gawliński, P.; Nikolay, R.; Goursot, C.; Lawo, S.; Chaurasia, B.; Herz, H.-M.; Kußler-Schneider, Y.; Ruppert, T.; Mayer, M.; Großhans, J. The Drosophila mitotic inhibitor Frühstart specifically binds to the hydrophobic patch of cyclins. EMBO Rep. 2007, 8, 490–496. [Google Scholar] [CrossRef]
- Gönczy, P.; Thomas, B.J.; DiNardo, S. Roughex is a dose-dependent regulator of the second meiotic division during Drosophila spermatogenesis. Cell 1994, 77, 1015–1025. [Google Scholar] [CrossRef]
- Hagting, A.; Jackman, M.; Simpson, K.; Pines, J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 1999, 9, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, R.; Ayeni, J.; Jin, Z.; Homola, E.; Campbell, S.D. Myt1 inhibition of cyclin A/Cdk1 is essential for fusome integrity and premeiotic centriole engagement in Drosophila spermatocytes. Mol. Biol. Cell 2016, 27, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, F.; Yakubovich, N.; O’Farrell, P.H. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr. Biol. 1997, 7, 488–499. [Google Scholar] [CrossRef]
- Moore, J.D.; Yang, J.; Truant, R.; Kornbluth, S. Nuclear import of Cdk/Cyclin complexes: Identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J. Cell Biol. 1999, 144, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, C.G.; Weis, K.; Morgan, D.O. Ran-independent nuclear import of cyclin B1–Cdc2 by importin β. Proc. Natl. Acad. Sci. USA 1999, 96, 7938–7943. [Google Scholar] [CrossRef]
- Guo, L.; Mohd, K.S.; Ren, H.; Xin, G.; Jiang, Q.; Clarke, P.R.; Zhang, C. Phosphorylation of importin-α1 by CDK1-cyclin B1 controls mitotic spindle assembly. J. Cell Sci. 2019, 132, jcs232314. [Google Scholar] [CrossRef]
- Toyoshima-Morimoto, F.; Taniguchi, E.; Shinya, N.; Iwamatsu, A.; Nishida, E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 2001, 410, 215–220. [Google Scholar] [CrossRef]
- Perry, J.A.; Kornbluth, S. Cdc25 and Wee1: Analogous opposites? Cell Div. 2007, 2, 12. [Google Scholar] [CrossRef]
- White-Cooper, H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010, 139, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Dumont, T.M.; Chatterjee, C.; Wasserman, S.A.; DiNardo, S. A novel eIF4G homolog, off-schedule, couples translational control to meiosis and differentiation in Drosophila spermatocytes. Development 2007, 134, 2851–2861. [Google Scholar] [CrossRef]
- Baker, C.C.; Fuller, M.T. Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog. Development 2007, 134, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Kachaner, D.; Garrido, D.; Mehsen, H.; Normandin, K.; Lavoie, H.; Archambault, V. Coupling of Polo kinase activation to nuclear localization by a bifunctional NLS is required during mitotic entry. Nat. Commun. 2017, 8, 1701. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Kemp, J.P., Jr.; Tarzia, M.; Deneke, V.E.; Marzluff, W.F.; Duronio, R.J.; Di Talia, S. CDK-regulated phase separation seeded by histone genes ensures precise growth and function of Histone Locus Bodies. Dev. Cell 2020, 54, 379–394. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazoe, K.; Inoue, Y.H. Cyclin B Export to the Cytoplasm via the Nup62 Subcomplex and Subsequent Rapid Nuclear Import Are Required for the Initiation of Drosophila Male Meiosis. Cells 2023, 12, 2611. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12222611
Yamazoe K, Inoue YH. Cyclin B Export to the Cytoplasm via the Nup62 Subcomplex and Subsequent Rapid Nuclear Import Are Required for the Initiation of Drosophila Male Meiosis. Cells. 2023; 12(22):2611. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12222611
Chicago/Turabian StyleYamazoe, Kanta, and Yoshihiro H. Inoue. 2023. "Cyclin B Export to the Cytoplasm via the Nup62 Subcomplex and Subsequent Rapid Nuclear Import Are Required for the Initiation of Drosophila Male Meiosis" Cells 12, no. 22: 2611. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12222611