Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation
Abstract
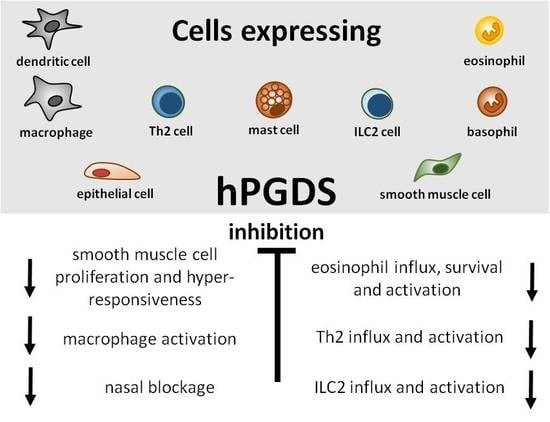
:1. Introduction
2. hPGDS Structure, Function and Regulation
3. Hematopoietic PGDS Expression in Leukocytes and Parenchymal Cells
3.1. Immune Cells
3.2. Parenchymal Cells
4. PGD2 Signaling as Therapeutic Target in Allergic Diseases
4.1. Allergic Asthma and Rhinitis
4.2. Atopic Dermatitis
4.3. Food Allergy and Gastrointestinal Allergic Disorder
4.4. Anaphylactic Reaction
5. Patents and Clinical Studies
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettipher, R. The roles of the prostaglandin D 2 receptors DP 1 and CRTH2 in promoting allergic responses. Br. J. Pharmacol. 2008, 153, 191–199. [Google Scholar]
- Marone, G.; Galdiero, M.R.; Pecoraro, A.; Pucino, V.; Criscuolo, G.; Triassi, M.; Varricchi, G. Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives. Expert Opin. Investig. Drugs 2018, 28, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Schuligoi, R.; Sturm, E.; Luschnig, P.; Konya, V.; Philipose, S.; Sedej, M.; Waldhoer, M.; Peskar, B.A.; Heinemann, A. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology 2010, 85, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-L.; Ricciotti, E.; Liang, X.; Grosser, T.; Grant, G.R.; FitzGerald, G.A. Lipocalin-Like Prostaglandin D Synthase but Not Hemopoietic Prostaglandin D Synthase Deletion Causes Hypertension and Accelerates Thrombogenesis in Mice. J. Pharmacol. Exp. Ther. 2018, 367, 425–432. [Google Scholar] [CrossRef]
- Yu, R.; Xiao, L.; Zhao, G.; Christman, J.W.; van Breemen, R.B. Competitive Enzymatic Interactions Determine the Relative Amounts of Prostaglandins E2 and D2. J. Pharmacol. Exp. Ther. 2011, 339, 716–725. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.; Leff, P.; Bolofo, M.L.; Kelly, M.G.; Robertson, A.D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989, 96, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad, H.; Kool, M.; Soullié, T.; Narumiya, S.; Trottein, F.; Hoogsteden, H.C.; Lambrecht, B.N. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 2007, 204, 357–367. [Google Scholar] [CrossRef]
- Spik, I.; Brenuchon, C.; Angeli, V.; Staumont, D.; Fleury, S.; Capron, M.; Trottein, F.; Dombrowicz, D. Activation of the Prostaglandin D2 Receptor DP2/CRTH2 Increases Allergic Inflammation in Mouse. J. Immunol. 2005, 174, 3703–3708. [Google Scholar] [CrossRef]
- Arimura, A.; Yasui, K.; Kishino, J.; Asanuma, F.; Hasegawa, H.; Kakudo, S.; Ohtani, M.; Arita, H. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J. Pharmacol. Exp. Ther. 2001, 298, 411–419. [Google Scholar]
- Jandl, K.; Stacher, E.; Bálint, Z.; Sturm, E.M.; Maric, J.; Peinhaupt, M.; Luschnig, P.; Aringer, I.; Fauland, A.; Konya, V.; et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J. Allergy Clin. Immunol. 2016, 137, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarashina, H.; Tsubosaka, Y.; Omori, K.; Aritake, K.; Nakagawa, T.; Hori, M.; Hirai, H.; Nakamura, M.; Narumiya, S.; Urade, Y.; et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J. Immunol. 2014, 192, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N.; Singh, A.; Wallace, J.L. Cyclooxygenase-2-derived prostaglandin D 2 is an early anti-inflammatory signal in experimental colitis. Am. J. Physiol. Liver Physiol. 2000, 279, G238–G244. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Aritake, K.; Tsubosaka, Y.; Maruyama, T.; Nakagawa, T.; Hori, M.; Hirai, H.; Nakamura, M.; Narumiya, S.; Urade, Y.; et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl. Acad. Sci. USA 2013, 110, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fujiwara, Y.; Yamada, R.; Fujii, W.; Hamabata, T.; Lee, M.Y.; Maeda, S.; Aritake, K.; Roers, A.; Sessa, W.C.; et al. Mast cell–derived prostaglandin D2attenuates anaphylactic reactions in mice. J. Allergy Clin. Immunol. 2017, 140, 630–632.e9. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Moroi, R.; Aritake, K.; Urade, Y.; Kanai, Y.; Sumi, K.; Yokozeki, H.; Hirai, H.; Nagata, K.; Hara, T.; et al. Prostaglandin D2 Plays an Essential Role in Chronic Allergic Inflammation of the Skin via CRTH2 Receptor. J. Immunol. 2006, 177, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, Y.; Kanaoka, Y.; Aritake, K.; Uodome, N.; Okazaki-Hatake, K.; Urade, Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J. Immunol. 2002, 168, 443–449. [Google Scholar] [CrossRef]
- Matsuoka, T.; Hirata, M.; Tanaka, H.; Takahashi, Y.; Murata, T.; Kabashima, K.; Sugimoto, Y.; Kobayashi, T.; Ushikubi, F.; Aze, Y.; et al. Prostaglandin D 2 as a mediator of allergic asthma. Science 2000, 287, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Pillinger, M.H. 15d-PGJ2: The anti-inflammatory prostaglandin? Clin. Immunol. 2005, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Egan, K.; Bell-Parikh, L.C.; FitzGerald, G.A. Activation of nuclear receptors by prostaglandins. Thromb. Res. 2003, 110, 311–315. [Google Scholar] [CrossRef]
- Jandl, K.; Heinemann, A. The therapeutic potential of CRTH2/DP2 beyond allergy and asthma. Prostaglandins Other Lipid Mediat. 2017, 3, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Narumiya, S. The DP receptor, allergic inflammation and asthma. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 187–194. [Google Scholar] [CrossRef]
- Urade, Y.; Eguchi, N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002, 68–69, 375–382. [Google Scholar] [CrossRef]
- Uchida, Y.; Urade, Y.; Mori, S.; Kohzuma, T. UV resonance Raman studies on the activation mechanism of human hematopoietic prostaglandin D2 synthase by a divalent cation, Mg2+. J. Inorg. Biochem. 2010, 104, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Irikura, D.; Okazaki, N.; Kinugasa, S.; Matsumura, H.; Uodome, N.; Yamamoto, M.; Kumasaka, T.; Miyano, M.; Kai, Y.; et al. Mechanism of metal activation of human hematopoietic prostaglandin D synthase. Nat Struct Mol Biol 2003, 10, 291–296. [Google Scholar] [CrossRef]
- Pinzar, E.; Miyano, M.; Kanaoka, Y.; Urade, Y.; Hayaishi, O. Structural basis of hematopoietic prostaglandin D synthase activity elucidated by site-directed mutagenesis. J. Biol. Chem. 2000, 275, 31239–31244. [Google Scholar] [CrossRef] [PubMed]
- Tippin, B.L.; Levine, A.J.; Materi, A.M.; Song, W.-L.; Keku, T.O.; Goodman, J.E.; Sansbury, L.B.; Das, S.; Dai, A.; Kwong, A.M.; et al. Hematopoietic prostaglandin D synthase (HPGDS): A high stability, Val187Ile isoenzyme common among African Americans and its relationship to risk for colorectal cancer. Prostaglandins Other Lipid Mediat. 2012, 97, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Aritake, K.; Kado, Y.; Inoue, T.; Miyano, M.; Urade, Y. Structural and functional characterization of HQL-79, an orally selective inhibitor of human hematopoietic prostaglandin D synthase. J. Biol. Chem. 2006, 281, 15277–15286. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, R.; Deng, J.; Zhao, Q.; Li, Y.; Joo, M.; van Breemen, R.B.; Christman, J.W.; Xiao, L. Pivotal role of reactive oxygen species in differential regulation of lipopolysaccharide-induced prostaglandins production in macrophages. Mol. Pharmacol. 2013, 83, 167–178. [Google Scholar] [CrossRef]
- Ishii, T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D 2 and E 2 production in macrophages in acute inflammation. Free Radic. Biol. Med. 2015, 88, 189–198. [Google Scholar] [CrossRef]
- Chatterjee, S.; Feinstein, S.I.; Dodia, C.; Sorokina, E.; Lien, Y.C.; Nguyen, S.; Debolt, K.; Speicher, D.; Fisher, A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011, 286, 11696–11706. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Sadikot, R.T.; Xiao, L.; Christman, J.W.; Freeman, M.L.; Chan, J.Y.; Oh, Y.K.; Blackwell, T.S.; Joo, M. Nrf2 is essential for the expression of lipocalin-prostaglandin D synthase induced by prostaglandin D2. Free Radic. Biol. Med. 2013, 65, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Luna-Gomes, T.; Magalhaes, K.G.; Mesquita-Santos, F.P.; Bakker-Abreu, I.; Samico, R.F.; Molinaro, R.; Calheiros, A.S.; Diaz, B.L.; Bozza, P.T.; Weller, P.F.; et al. Eosinophils as a Novel Cell Source of Prostaglandin D2: Autocrine Role in Allergic Inflammation. J. Immunol. 2011, 187, 6518–6526. [Google Scholar] [CrossRef] [PubMed]
- Jowsey, I.R.; Thomson, A.M.; Flanagan, J.U.; Murdock, P.R.; Moore, G.B.; Meyer, D.J.; Murphy, G.J.; Smith, S.A.; Hayes, J.D. Mammalian class Sigma glutathione S-transferases: catalytic properties and tissue-specific expression of human and rat GSH-dependent prostaglandin D2 synthases. Biochem. J. 2001, 359, 507–516. [Google Scholar] [PubMed] [Green Version]
- Balzar, S.; Fajt, M.L.; Comhair, S.A.A.; Erzurum, S.C.; Bleecker, E.; Busse, W.W.; Castro, M.; Gaston, B.; Israel, E.; Schwartz, L.B.; et al. Mast cell phenotype, location, and activation in severe asthma: Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2011, 183, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [Green Version]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef]
- Lewis, R.A.; Soter, N.A.; Diamond, P.T.; Austen, K.F.; Oates, J.A.; Roberts, L.J. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J. Immunol. 1982, 129, 1627–1631. [Google Scholar]
- Baothman, B.K.; Smith, J.; Kay, L.J.; Suvarna, S.K.; Peachell, P.T. Prostaglandin D2generation from human lung mast cells is catalysed exclusively by cyclooxygenase-1. Eur. J. Pharmacol. 2018, 819, 225–232. [Google Scholar] [CrossRef]
- Maeda, S.; Nakamura, T.; Harada, H.; Tachibana, Y.; Aritake, K.; Shimosawa, T.; Yatomi, Y.; Murata, T. Prostaglandin D2metabolite in urine is an index of food allergy. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Maeda, S.; Horiguchi, K.; Maehara, T.; Aritake, K.; Choi, B.I.; Iwakura, Y.; Urade, Y.; Murata, T. PGD2deficiency exacerbates food antigen-induced mast cell hyperplasia. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Fujiwara, T.; Sugata, Y.; Gotoh, D.; Masaoka, Y.; Sogo, M.; Tanimoto, W.; Yamamoto, M.; Matsumoto, R.; Eguchi, N.; et al. Presence and characterization of prostaglandin D2–related molecules in nasal mucosa of patients with allergic rhinitis. Am. J. Rhinol. 2006, 20, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Aritake, K.; Matsumoto, S.; Kamauchi, S.; Nakagawa, T.; Hori, M.; Momotani, E.; Urade, Y.; Ozaki, H. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 19802–19807. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinhaupt, M.; Sturm, E.M.; Heinemann, A. Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets. Front. Med. 2017, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lamkhioued, B.; Aldebert, D.; Gounni, A.S.; Delaporte, E.; Goldman, M.; Capron, A.; Capron, M. Synthesis of cytokines by eosinophils and their regulation. Int. Arch. Allergy Immunol. 1995, 107, 122–123. [Google Scholar] [CrossRef]
- Peinhaupt, M.; Roula, D.; Theiler, A.; Sedej, M.; Schicho, R.; Marsche, G.; Sturm, E.M.; Sabroe, I.; Rothenberg, M.E.; Heinemann, A. DP1 receptor signaling prevents the onset of intrinsic apoptosis in eosinophils and functions as a transcriptional modulator. J. Leukoc. Biol. 2018, 104, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Radnai, B.; Sturm, E.M.; Stancic, A.; Jandl, K.; Labocha, S.; Ferreirós, N.; Grill, M.; Hasenoehrl, C.; Gorkiewicz, G.; Marsche, G.; et al. Eosinophils contribute to intestinal inflammation via chemoattractant receptor-homologous molecule expressed on Th2 Cells, CRTH2, in experimental crohn’s disease. J. Crohn’s Colitis 2016, 10, 1087–1095. [Google Scholar] [CrossRef]
- Feng, X.; Ramsden, M.K.; Negri, J.; Baker, M.G.; Payne, S.C.; Borish, L.; Steinke, J.W. Eosinophil production of PGD2 in Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. 2016, 138, 1089–1097. [Google Scholar] [CrossRef]
- Miyake, K.; Karasuyama, H. Emerging roles of basophils in allergic inflammation. Allergol. Int. 2017, 66, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Koshino, T.; Arai, Y.; Miyamoto, Y.; Sano, Y.; Itami, M.; Teshima, S.; Hirai, K.; Takaishi, T.; Ito, K.; Morita, Y. Airway basophil and mast cell density in patients with bronchial asthma: relationship to bronchial hyperresponsiveness. J. Asthma 1996, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kepley, C.L.; McFeeley, P.J.; Oliver, J.M.; Lipscomb, M.F. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Tanaka, K.; Yoshie, O.; Ogawa, K.; Kenmotsu, K.; Takamori, Y.; Ichimasa, M.; Sugamura, K.; Nakamura, M.; Takano, S.; et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001, 193, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Ito, Y.; Miyagishi, C.; Yokozeki, H. Basophils Infiltrate Skin Lesions of Eosinophilic Pustular Folliculitis (Ofuji’s Disease). Acta Derm. Venereol. 2011, 91, 371–372. [Google Scholar] [CrossRef]
- Ugajin, T.; Satoh, T.; Kanamori, T.; Aritake, K.; Urade, Y.; Yokozeki, H. FcεRI, but Not FcγR, Signals Induce Prostaglandin D2 and E2 Production from Basophils. Am. J. Pathol. 2011, 179, 775–782. [Google Scholar] [CrossRef]
- Pellefigues, C.; Dema, B.; Lamri, Y.; Saidoune, F.; Chavarot, N.; Lohéac, C.; Pacreau, E.; Dussiot, M.; Bidault, C.; Marquet, F.; et al. Prostaglandin D2amplifies lupus disease through basophil accumulation in lymphoid organs. Nat. Commun. 2018, 9, 725. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Arebro, J.; Ekstedt, S.; Hjalmarsson, E.; Winqvist, O.; Kumlien Georén, S.; Cardell, L.O. A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Polak, D.; Samadi, N.; Vizzardelli, C.; Acosta, G.S.; Rosskopf, S.; Steinberger, P.; Jahn-Schmid, B.; Bohle, B. Neutrophils promote T-cell-mediated inflammation in allergy. J. Allergy Clin. Immunol. 2018, 143, 1923–1925. [Google Scholar] [CrossRef]
- Polak, D.; Hafner, C.; Briza, P.; Kitzmüller, C.; Elbe-Bürger, A.; Samadi, N.; Gschwandtner, M.; Pfützner, W.; Zlabinger, G.J.; Jahn-Schmid, B.; et al. A novel role for neutrophils in IgE-mediated allergy: Evidence for antigen presentation in late-phase reactions. J. Allergy Clin. Immunol. 2018, 148, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Rankin, G.; Blomberg, A.; Smed-Sörensen, A. Human lung mononuclear phagocytes in health and disease. Front. Immunol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Urade, Y.; Ujihara, M.; Horiguchi, Y.; Ikai, K.; Hayaishi, O. The major source of endogenous prostaglandin D2 production is likely antigen-presenting cells. Localization of glutathione-requiring prostaglandin D synthetase in histiocytes, dendritic, and Kupffer cells in various rat tissues. J. Immunol. 1989, 143, 2982–2989. [Google Scholar] [PubMed]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef]
- Kowal, K.; Møller, H.J.; DuBuske, L.M.; Moestrup, S.K.; Bodzenta-Lukaszyk, A. Differential expression of monocyte CD163 in single and dual-asthmatic responders during allergen-induced bronchoconstriction. Clin. Exp. Allergy 2006, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Collier, F.; Naselli, G.; Saffery, R.; Tang, M.L.; Allen, K.J.; Ponsonby, A.-L.; Harrison, L.C.; Vuillermin, P. Cord blood monocyte–derived inflammatory cytokines suppress IL-2 and induce nonclassic “T H 2-type” immunity associated with development of food allergy. Sci. Transl. Med. 2016, 8, 321ra8. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Bernal, A.L.; Starkey, P.M. Prostaglandin production by human peripheral blood monocytes changes with in vitro differentiation. Prostaglandins 1996, 51, 339–349. [Google Scholar] [CrossRef]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Draijer, C.; Boorsma, C.E.; Robbe, P.; Timens, W.; Hylkema, M.N.; Ten Hacken, N.H.; van den Berge, M.; Postma, D.S.; Melgert, B.N. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J. Allergy Clin. Immunol. 2017, 140, 280–283.e3. [Google Scholar] [CrossRef]
- Kumar, S.; Dwivedi, P.D.; Das, M.; Tripathi, A. Macrophages in food allergy: An enigma. Mol. Immunol. 2013, 56, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Kasraie, S.; Werfel, T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediators Inflamm. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ornatowska, M.; Zhao, G.; Cao, H.; Yu, R.; Deng, J.; Li, Y.; Zhao, Q.; Sadikot, R.T.; Christman, J.W. Lipopolysaccharide-Induced Expression of Microsomal Prostaglandin E Synthase-1 Mediates Late-Phase PGE2 Production in Bone Marrow Derived Macrophages. PLoS ONE 2012, 7, e50244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Lee, Y.J.; Choi, Y.H.; Park, Y.M.; Kang, J.L. Macrophages programmed by apoptotic cells inhibit epithelial-mesenchymal transition in lung alveolar epithelial cells via PGE2, PGD2, and HGF. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Shen, Y.; Liu, G.; Zuo, S.; Ji, Y.; Lu, A.; Nakamura, M.; Lazarus, M.; Stratakis, C.A.; Breyer, R.M.; et al. PKA regulatory IIα subunit is essential for PGD 2 -mediated resolution of inflammation. J. Exp. Med. 2016, 213, 2209–2226. [Google Scholar] [CrossRef] [PubMed]
- Virtue, S.; Masoodi, M.; de Weijer, B.A.M.; van Eijk, M.; Mok, C.Y.L.; Eiden, M.; Dale, M.; Pirraco, A.; Serlie, M.J.; Griffin, J.L.; et al. Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans. Int. J. Obes. 2015, 39, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Henkel, F.D.R.; Friedl, A.; Haid, M.; Thomas, D.; Bouchery, T.; Haimerl, P.; de los Reyes Jiménez, M.; Alessandrini, F.; Schmidt-Weber, C.B.; Harris, N.L.; et al. House dust mite drives pro-inflammatory eicosanoid reprogramming and macrophage effector functions. Allergy 2018, 74, 1090–1101. [Google Scholar] [CrossRef]
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic Cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. Int. J. Mol. Sci. 2017, 18, 1491. [Google Scholar] [CrossRef]
- Gosset, P.; Pichavant, M.; Faveeuw, C.; Bureau, F.; Tonnel, A.B.; Trottein, F. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: Impact on the T cell response. Eur. J. Immunol. 2005, 35, 1491–1500. [Google Scholar] [CrossRef]
- Tumala, B.; Phelps, K.R.; Zhang, S.; Bhattacharya, S.; Shornick, L.P. Prostaglandin D 2 Levels Regulate CD103 + Conventional Dendritic Cell Activation in Neonates During Respiratory Viral Infection. Viral Immunol. 2018, 31, 658–667. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.H.; Murray, F.; Li, X.; Choi, S.S.; Broide, D.H.; Corr, M.; Lee, J.; Webster, N.J.G.; Insel, P.A.; et al. Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proc. Natl. Acad. Sci. USA 2015, 112, 1529–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimura, C.; Satoh, T.; Igawa, K.; Aritake, K.; Urade, Y.; Nakamura, M.; Yokozeki, H. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am. J. Pathol. 2010, 176, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Moghaddami, M.; Ranieri, E.; James, M.; Fletcher, J.; Cleland, L.G. Prostaglandin D(2) in inflammatory arthritis and its relation with synovial fluid dendritic cells. Mediators Inflamm. 2013, 2013, 329494. [Google Scholar] [CrossRef] [PubMed]
- Maric, J.; Ravindran, A.; Mazzurana, L.; Van Acker, A.; Rao, A.; Kokkinou, E.; Ekoff, M.; Thomas, D.; Fauland, A.; Nilsson, G.; et al. Cytokine-induced endogenous production of PGD2 is essential for human ILC2 activation. J. Allergy Clin. Immunol. 2018, 143, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. 2000, 105, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Ito, T.; Wang, Y.-H.; Homey, B.; Watanabe, N.; Martin, R.; Barnes, C.J.; McIntyre, B.W.; Gilliet, M.; Kumar, R.; et al. Maintenance and Polarization of Human TH2 Central Memory T Cells by Thymic Stromal Lymphopoietin-Activated Dendritic Cells. Immunity 2006, 24, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seumois, G.; Zapardiel-Gonzalo, J.; White, B.; Singh, D.; Schulten, V.; Dillon, M.; Hinz, D.; Broide, D.H.; Sette, A.; Peters, B.; et al. Transcriptional Profiling of Th2 Cells Identifies Pathogenic Features Associated with Asthma. J. Immunol. 2016, 197, 655–664. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogawa, K.; Sugamura, K.; Nakamura, M.; Takano, S.; Nagata, K. Cutting Edge: Differential Production of Prostaglandin D2 by Human Helper T Cell Subsets. J. Immunol. 2000, 164, 2277–2280. [Google Scholar] [CrossRef]
- Mitson-Salazar, A.; Yin, Y.; Wansley, D.L.; Young, M.; Bolan, H.; Arceo, S.; Ho, N.; Koh, C.; Milner, J.D.; Stone, K.D.; et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human TH2 cell subpopulation with enhanced function. J. Allergy Clin. Immunol. 2016, 137, 907–918. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Monticelli, L.A.; Tran, S.V.; Alenghat, T.; Osborne, L.C.; Thome, J.J.; Willis, C.; Budelsky, A.; Farber, D.L.; Artis, D. The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015, 8, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Maric, J.; Ravindran, A.; Mazzurana, L.; Björklund, Å.K.; Van Acker, A.; Rao, A.; Friberg, D.; Dahlén, S.-E.; Heinemann, A.; Konya, V.; et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J. Allergy Clin. Immunol. 2018, 141, 1761–1773.e6. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Lajoie, S. Epithelial Cell Regulation of Allergic Diseases. Curr. Allergy Asthma Rep. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Werder, R.B.; Lynch, J.P.; Simpson, J.C.; Zhang, V.; Hodge, N.H.; Poh, M.; Forbes-Blom, E.; Kulis, C.; Smythe, M.L.; Upham, J.W.; et al. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN- production. Sci. Transl. Med. 2018, 10, eaao0052. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, B.; Gielicz, A.; Plutecka, H.; Hubalewska-Mazgaj, M.; Mastalerz, L.; Bochenek, G.; Soja, J.; Januszek, R.; Aab, A.; Musial, J.; et al. Th2-type cytokine induced mucous metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Legraverend, C.; Jay, P. The intestinal epithelium tuft cells: specification and function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Kano, R.; Ishikawa, T.; Watanabe, S. The antimycotic drugs itraconazole and terbinafine hydrochloride induce the production of human β-defensin-3 in human keratinocytes. Immunobiology 2011, 216, 497–504. [Google Scholar] [CrossRef]
- Konya, V.; Üllen, A.; Kampitsch, N.; Theiler, A.; Philipose, S.; Parzmair, G.P.; Marsche, G.; Peskar, B.A.; Schuligoi, R.; Sattler, W.; et al. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J. Allergy Clin. Immunol. 2013, 131, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Asosingh, K.; Swaidani, S.; Aronica, M.; Erzurum, S.C. Th1- and Th2-Dependent Endothelial Progenitor Cell Recruitment and Angiogenic Switch in Asthma. J. Immunol. 2014, 178, 6482–6494. [Google Scholar] [CrossRef]
- Asosingh, K.; Weiss, K.; Queisser, K.; Wanner, N.; Yin, M.; Aronica, M.; Erzurum, S. Endothelial cells in the innate response to allergens and initiation of atopic asthma. J. Clin. Invest. 2018, 128, 3116–3128. [Google Scholar] [CrossRef] [Green Version]
- Taba, Y.; Sasaguri, T.; Miyagi, M.; Abumiya, T.; Miwa, Y.; Ikeda, T.; Mitsumata, M. Fluid shear stress induces lipocalin-type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ. Res. 2000, 86, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Morikawa, T.; Kunita, A.; Nakamura, T.; Aritake, K.; Urade, Y.; Fukayama, M.; Murata, T. Lipocalin-type prostaglandin D synthase-derived PGD 2 attenuates malignant properties of tumor endothelial cells. J. Pathol. 2018, 244, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Suto, W.; Sakai, H. Augmented Pla2g4c/Ptgs2/Hpgds axis in bronchial smooth muscle tissues of experimental asthma. PLoS ONE 2018, 13, e0202623. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zuo, S.; Tang, J.; Zuo, C.; Jia, D.; Liu, Q.; Liu, G.; Zhu, Q.; Wang, Y.; Zhang, J.; et al. Inhibition of CRTH2-mediated Th2 activation attenuates pulmonary hypertension in mice. J. Exp. Med. 2018, 215, 2175–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakiela, B.; Gielicz, A.; Plutecka, H.; Hubalewska, M.; Mastalerz, L.; Bochenek, G.; Soja, J.; Januszek, R.; Musial, J.; Sanak, M. Eicosanoid biosynthesis during mucociliary and mucous metaplastic differentiation of bronchial epithelial cells. Prostaglandins Other Lipid Mediat. 2013, 106, 116–123. [Google Scholar] [CrossRef]
- Obata, T.; Nagakura, T.; Masaki, T.; Maekawa, K.; Yamashita, K. Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclo-oxygenase-2 in cultured human mast cells. Clin. Exp. Allergy 1999, 29, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Björklund, Å.K.; Forkel, M.; Picelli, S.; Konya, V.; Theorell, J.; Friberg, D.; Sandberg, R.; Mjösberg, J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016, 17, 451–460. [Google Scholar] [CrossRef]
- Barnes, P.J. New therapies for asthma: is there any progress? Trends Pharmacol. Sci. 2010, 31, 335–343. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yoshikawa, M.; Otori, N.; Haruna, S.; Moriyama, H. Correlation between the Prostaglandin D2/E2 Ratio in Nasal Polyps and the Recalcitrant Pathophysiology of Chronic Rhinosinusitis Associated with Bronchial Asthma. Allergol. Int. 2008, 57, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Fajt, M.L.; Gelhaus, S.L.; Freeman, B.; Uvalle, C.E.; Trudeau, J.B.; Holguin, F.; Wenzel, S.E. Prostaglandin D2 pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J. Allergy Clin. Immunol. 2013, 131, 1504–1512.e12. [Google Scholar] [CrossRef] [Green Version]
- al Jarad, N.; Hui, K.; Barnes, N. Effects of a thromboxane receptor antagonist on prostaglandin D2 and histamine induced bronchoconstriction in man. Br. J. Clin. Pharmacol. 1994, 37, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.L.; Freezer, N.J.; Ritter, W.; O’Toole, S.; Howarth, P.H. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur. Respir. J. 1995, 8, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.A.; Birrell, M.A.; Adcock, J.J.; Wortley, M.A.; Dubuis, E.D.; Bonvini, S.J.; Grace, M.S.; Belvisi, M.G. Prostaglandin D2 and the role of the DP1, DP2 and TP receptors in the control of airway reflex events. Eur. Respir. J. 2015, 45, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Kostenis, E.; Ulven, T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol. Med. 2006, 12, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.N.; Labzin, L.; Bourne, G.T.; Fukunishi, H.; Weber, J.E.; Sweet, M.J.; Smythe, M.L.; Flanagan, J.U. Development and Characterization of New Inhibitors of the Human and Mouse Hematopoietic Prostaglandin D2 Synthases. J. Med. Chem. 2010, 53, 5536–5548. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Radovanovic, D. Prostaglandin D2 receptor antagonists in early development as potential therapeutic options for asthma. Expert Opin. Investig. Drugs 2016, 25, 1083–1092. [Google Scholar] [CrossRef]
- Kupczyk, M.; Kuna, P. Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs 2017, 77, 1281–1294. [Google Scholar] [CrossRef] [Green Version]
- James, K.M.; Gebretsadik, T.; Escobar, G.J.; Wu, P.; Carroll, K.N.; Li, S.X.; Walsh, E.M.; Mitchel, E.F.; Sloan, C.; Hartert, T.V. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J. Allergy Clin. Immunol. 2013, 132, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Kajiwara, D.; Aoyagi, H.; Shigeno, K.; Togawa, M.; Tanaka, K.; Inagaki, N.; Miyoshi, K. Role of hematopoietic prostaglandin D synthase in biphasic nasal obstruction in guinea pig model of experimental allergic rhinitis. Eur. J. Pharmacol. 2011, 667, 389–395. [Google Scholar] [CrossRef]
- Nabe, T.; Kuriyama, Y.; Mizutani, N.; Shibayama, S.; Hiromoto, A.; Fujii, M.; Tanaka, K.; Kohno, S. Inhibition of hematopoietic prostaglandin D synthase improves allergic nasal blockage in guinea pigs. Prostaglandins Other Lipid Mediat. 2011, 95, 27–34. [Google Scholar] [CrossRef]
- Flohr, C.; Mann, J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014, 69, 3–16. [Google Scholar] [CrossRef]
- Hajar, T.; Gontijo, J.R.V.; Hanifin, J.M. New and developing therapies for atopic dermatitis. An. Bras. Dermatol. 2018, 93, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.A.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Perry, T.T.; Pesek, R.D. Clinical Manifestations of Food Allergy. Pediatr. Ann. 2013, 42, e106–e111. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E.; Wang, Y.-H. Hematopoietic prostaglandin D synthase: Linking pathogenic effector CD4+ TH2 cells to proeosinophilic inflammation in patients with gastrointestinal allergic disorders. J. Allergy Clin. Immunol. 2016, 137, 919–921. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, X.; Yu, S. Prostaglandin D2 receptor D-type prostanoid receptor 2 mediates eosinophil trafficking into the esophagus. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2014, 27, 601–606. [Google Scholar] [CrossRef]
- Straumann, A.; Hoesli, S.; Bussmann, C.; Stuck, M.; Perkins, M.; Collins, L.P.; Payton, M.; Pettipher, R.; Hunter, M.; Steiner, J.; et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013, 68, 375–385. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Frew, A.J.; Ansotegui, I.J.; Bochner, B.S.; Golden, D.B.K.; Finkelman, F.D.; Leung, D.Y.M.; Lotvall, J.; Marone, G.; Metcalfe, D.D.; et al. Risk assessment in anaphylaxis: current and future approaches. J. Allergy Clin. Immunol. 2007, 120, S2–24. [Google Scholar] [CrossRef]
- Ono, E.; Taniguchi, M.; Mita, H.; Fukutomi, Y.; Higashi, N.; Miyazaki, E.; Kumamoto, T.; Akiyama, K. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin. Exp. Allergy 2009, 39, 72–80. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tsubosaka, Y.; Hori, M.; Narumiya, S.; Ozaki, H.; Murata, T. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 565–571. [Google Scholar] [CrossRef]
- Sanofi, Paris (FR). Pyrimidine Hydrazide Compounds as PGDS Inhibitors. U.S. Patent 8258,130 B2, 4 September 2012. [Google Scholar]
- Sanofi, Paris (FR). Phenyloxadialzole Derivatives as PGDS Inhibitors. U.S. Patent 9,937,175 B2, 10 April 2018. [Google Scholar]
- Astex Therapeutics Limited (UK); GlaxoSmithKline Intelectual (UK). Quinoline-3-Carboxamides as h-pgds Inhibitors. WO Patent 2017/103851 A1, 22 June 2017. [Google Scholar]
- Zai Lab Pty. Ltd. Study of the Tolerability and Pharmacokinetic of ZL-2102 With an Investigation of Food Effect in Healthy Male Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT02397005 (accessed on 7 March 2019).
- Takeshita, E.; Komaki, H.; Shimizu-Motohashi, Y.; Ishiyama, A.; Sasaki, M.; Takeda, S. A phase I study of TAS-205 in patients with Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2018, 5, 1338–1349. [Google Scholar] [CrossRef] [Green Version]
- Farhat, A.; Philibert, P.; Sultan, C.; Poulat, F.; Boizet-Bonhoure, B. Hematopoietic-Prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology. J. Ovarian Res. 2011, 4, 3. [Google Scholar] [CrossRef]
- Cahill, K.N.; Bensko, J.C.; Boyce, J.A.; Laidlaw, T.M. Prostaglandin D2: A dominant mediator of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2015, 135, 245–252. [Google Scholar] [CrossRef]



| Cell Type | Species | hPGDS Expression | Stimuli | PGD2 Release | Ref. | |
|---|---|---|---|---|---|---|
| Parenchymal Cells | Epithelial cells | ms hu | mRNA, protein mRNA, protein | RSV infection Unstimulated IL-13, IL-4 | 1.2 ng/mL 100 pg/mL 25–50 pg/mL | [43,93] [93] [105] |
| Endothelial cells | hu | LPGDS-mRNA, protein LPGDS-protein | sheer stress tumor CM | 150 pg/mL 600 pg/mL | [101] [102] | |
| Smooth muscle cells | ms | mRNA, protein | OVA lung inflammation | [103] | ||
| Keratinocytes | hu | protein | antimycotics | 8 ng/106 cells | [97] | |
| Immune Cells | Monocytes | hu | unstimulated | 66 fmol/106 cells | [67] | |
| Macrophages | ms | protein mRNA | LPS zymosan A -(peritoneal lavage) | 20 ng/mL 800 pg/mouse | [29,73] [75] | |
| hu | mRNA | IL-4, HDM | 400 pg/mL | [77] | ||
| rat | protein | [11,63] | ||||
| Dendritic cells | hu | mRNA, protein | LPS, INF-γ | 250 pg/106 cells | [82] | |
| rat, h | protein | [63,83] | ||||
| Eosinophils | ms hu | mRNA, protein mRNA, protein | eotaxin/A23187 eotaxin/A23187 | 15 pg/2 × 106 cells 15–80 pg/2 × 106 | [33] | |
| hu | mRNA, protein | Lysin-Aspirin | 1.5 ng/105 cells | [50] | ||
| Neutrophils | ms | protein | [14] | |||
| Basophils | ms hu | mRNA, protein protein | TNP-OVA (IgE) IL-3, anti IgE | 700 pg/5 × 105 cells 100 pg/5 × 105 cells | [56] [56,57] | |
| Mast cells | hu rat | anti-IgE anti-IgE anti-IgE | 40 ng/106 cells 1 ng/104 cells 13 ng/106 cells | [39] [106] [39] | ||
| ms | protein | [41,42,44] | ||||
| ILC2 | hu | mRNA mRNA | IL-33, IL-25, TSLP | 1.5 ng/mL | [84] [107] | |
| Th2 cells | hu | mRNA, protein | OKT3, KOLT-2 | 30 ng/5 × 105 | [88] | |
| hu | protein | PMA, ionomycin | 70 pg/mL | [89] |
| Inhibitor | Chemical Structure | Company | Cell Type/Disease | Concentration | Ref. |
|---|---|---|---|---|---|
| HQL-79 |  | Cayman Chemicals | BMDM, eosinophils; OVA-induced lung inflammation | 5–100 µM 10 mg/kg | [29,33] [28] |
| TFC-007 |  | Tocris | nasal blockage in guinea pigs | 30 mg/kg | [120] |
| HPGDS inhibitor I |  | Cayman Chemicals | basophils; murine lupus model | 5 mg/kg | [57] |
| TAS-204 |  | Taiho Pharmaceutical Co. Ltd. | nasal blockage in guinea pigs | 15–30 mg/kg | [119] |
| TAS-205 | not reported | Taiho Pharmaceutical Co. Ltd. | Duchenne‘s muscular dystrophy (Phase I) | 1.67–13.33 mg/kg/dose | [136] |
| KMN-698 | not reported | Sanofi Aventis (Cayman Chemicals) | ILC-2 | 100 nM | [84] |
| ZL-2102 | not reported | Zai Lab Pty. Ltd./Sanofi | healthy males (Phase I) | 5 to 750 mg | [135] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittchen, S.; Heinemann, A. Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation. Cells 2019, 8, 619. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060619
Rittchen S, Heinemann A. Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation. Cells. 2019; 8(6):619. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060619
Chicago/Turabian StyleRittchen, Sonja, and Akos Heinemann. 2019. "Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation" Cells 8, no. 6: 619. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060619