Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation
Abstract
:1. Introduction
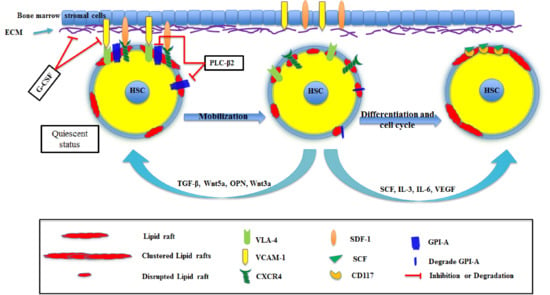
2. HSC Mobilization and Homing
3. HSC Differentiation
4. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Simons, K.; Van Meer, G. Lipid sorting in epithelial cells. Biochemistry 1988, 27, 6197–6202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodin, S.; Soulet, C.; Tronchere, H.; Sie, P.; Gachet, C.; Plantavid, M.; Payrastre, B. Integrin-dependent interaction of lipid rafts with the actin cytoskeleton in activated human platelets. J. Cell Sci. 2005, 118, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, R.; Mayor, S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 1998, 394, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Simons, K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005, 118, 1099–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid rafts: Heterogeneity on the high seas. Biochem. J. 2004, 378, 281–292. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef] [Green Version]
- Korade, Z.; Kenworthy, A.K. Lipid rafts, cholesterol, and the brain. Neuropharmacology 2008, 55, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Dharani, K. Chapter 7—Molecular-Grid Model. In The Biology of Thought; Dharani, K., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 123–142. [Google Scholar] [CrossRef]
- Oh, P.; Schnitzer, J.E. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol. Biol. Cell 2001, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C.; Cherukuri, A.; Dykstra, M.; Malapati, S.; Sproul, T.; Chen, M.R.; Pierce, S.K. Floating the raft hypothesis: The roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 2001, 13, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2002, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, M.; Cherukuri, A.; Sohn, H.W.; Tzeng, S.J.; Pierce, S.K. Location is everything: Lipid rafts and immune cell signaling. Annu. Rev. Immunol. 2003, 21, 457–481. [Google Scholar] [CrossRef]
- Cahuzac, N.; Baum, W.; Kirkin, V.; Conchonaud, F.; Wawrezinieck, L.; Marguet, D.; Janssen, O.; Zörnig, M.; Hueber, A.O. Fas ligand is localized to membrane rafts, where it displays increased cell death-inducing activity. Blood 2006, 107, 2384–2391. [Google Scholar] [CrossRef]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef]
- Perez-Chacon, G.; Vargas, J.A.; Jorda, J.; Alvarez, N.; Martin-Donaire, T.; Rosado, S.; Losada-Fernandez, I.; Rebolleda, N.; Perez-Aciego, P. CD5 does not regulate the signaling triggered through BCR in B cells from a subset of B-CLL patients. Leuk. Lymphoma 2007, 48, 147–157. [Google Scholar] [CrossRef]
- Cragg, M.S.; Morgan, S.M.; Chan, H.T.; Morgan, B.P.; Filatov, A.V.; Johnson, P.W.; French, R.R.; Glennie, M.J. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 2003, 101, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucero, H.; Gae, D.; Taccioli, G.E. Novel localization of the DNA-PK complex in lipid rafts: A putative role in the signal transduction pathway of the ionizing radiation response. J. Biol. Chem. 2003, 278, 22136–22143. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.A.; Ribon, V.; Kanzaki, M.; Thurmond, D.C.; Mora, S.; Shigematsu, S.; Bickel, P.E.; Pessin, J.E.; Saltiel, A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 2000, 407, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lucero, H.A.; Robbins, P.W. Lipid rafts-protein association and the regulation of protein activity. Arch. Biochem. Biophys. 2004, 426, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Carpentier, J.L.; Sawano, F.; Gorden, P.; Orci, L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc. Natl. Acad. Sci. USA 1987, 84, 7957–7961. [Google Scholar] [CrossRef] [PubMed]
- Stang, E.; Kartenbeck, J.; Parton, R.G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 1997, 8, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Greicius, G.; Westerberg, L.; Davey, E.J.; Buentke, E.; Scheynius, A.; Thyberg, J.; Severinson, E. Microvilli structures on B lymphocytes: Inducible functional domains? Int. Immunol. 2004, 16, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Oikawa, T. Membrane lipids in invadopodia and podosomes: Key structures for cancer invasion and metastasis. Oncotarget 2010, 1, 320–328. [Google Scholar] [CrossRef]
- Nicolson, G.L. Cell Membrane Fluid–Mosaic Structure and Cancer Metastasis. Cancer Res. 2015, 75, 1169–1176. [Google Scholar] [CrossRef]
- Staubach, S.; Hanisch, F.G. Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev. Proteom. 2011, 8, 263–277. [Google Scholar] [CrossRef]
- Hannaoui, S.; Shim, S.Y.; Cheng, Y.C.; Corda, E.; Gilch, S. Cholesterol Balance in Prion Diseases and Alzheimer’s Disease. Viruses 2014, 6, 4505–4535. [Google Scholar] [CrossRef] [PubMed]
- Janas, E.; Priest, R.; Wilde, J.I.; White, J.H.; Malhotra, R. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin. Exp. Immunol. 2005, 139, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Gajate, C.; Mollinedo, F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood 2007, 109, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.; Poblenz, A.; Haridas, V.; Zhang, C.; Duvic, M.; Gutterman, J. Triterpenoid electrophiles (avicins) suppress heat shock protein-70 and x-linked inhibitor of apoptosis proteins in malignant cells by activation of ubiquitin machinery: Implications for proapoptotic activity. Clin. Cancer Res. 2005, 11, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Reis-Sobreiro, M.; Gajate, C.; Mollinedo, F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 2009, 28, 3221–3234. [Google Scholar] [CrossRef] [Green Version]
- Pommier, A.J.; Alves, G.; Viennois, E.; Bernard, S.; Communal, Y.; Sion, B.; Marceau, G.; Damon, C.; Mouzat, K.; Caira, F.; et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene 2010, 29, 2712–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, B.; Gniadecki, R.; Gajkowska, B. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp. Dermatol. 2005, 14, 266–272. [Google Scholar] [CrossRef]
- Li, H.Y.; Appelbaum, F.R.; Willman, C.L.; Zager, R.A.; Banker, D.E. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood 2003, 101, 3628–3634. [Google Scholar] [CrossRef] [Green Version]
- Mineo, C.; James, G.L.; Smart, E.J.; Anderson, R.G. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 1996, 271, 11930–11935. [Google Scholar] [CrossRef]
- Liu, P.; Ying, Y.; Ko, Y.G.; Anderson, R.G. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J. Biol. Chem. 1996, 271, 10299–10303. [Google Scholar] [CrossRef]
- Nel, A.E. T-cell activation through the antigen receptor. Part 1: Signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 2002, 109, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.H.; Murray, F.; Insel, P.A. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. In Protein-Protein Interactions as New Drug Targets; Springer: Berlin/Heidelberg, Germany, 2008; pp. 167–184. [Google Scholar] [CrossRef]
- Ivanovs, A.; Rybtsov, S.; Anderson, R.A.; Turner, M.L.; Medvinsky, A. Identification of the Niche and Phenotype of the First Human Hematopoietic Stem Cells. Stem Cell Rep. 2014, 2, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, D.E.; Wagers, A.J.; Gulati, A.P.; Johnson, F.L.; Weissman, I.L. Physiological Migration of Hematopoietic Stem and Progenitor Cells. Science 2001, 294, 1933–1936. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A. Cellular and Molecular Immunology, 5th ed.; Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
- Dzierzak, E.; Speck, N.A. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat. Immunol. 2008, 9, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Medvinsky, A.; Rybtsov, S.; Taoudi, S. Embryonic origin of the adult hematopoietic system: Advances and questions. Development 2011, 138, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Lemischka, I.R. Microenvironmental regulation of hematopoietic stem cells. Stem Cells 2009, 15, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Sagar, B.M.M.; Rentala, S.; Gopal, P.N.V.; Sharma, S.; Mukhopadhyay, A. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem. Biophys. Res. Commun. 2006, 350, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.J.; Tanentzapf, G. Integrin-mediated adhesion and stem-cell-niche interactions. Cell Tissue Res. 2009, 339, 121. [Google Scholar] [CrossRef] [PubMed]
- Kurth, I.; Franke, K.; Pompe, T.; Bornhäuser, M.; Werner, C. Hematopoietic stem and progenitor cells in adhesive microcavities. Integr. Biol. 2009, 1, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2009, 339, 269. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Price, L.S.; Alderson, N.B.; Ren, X.-D.; Schwartz, M.A. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000, 19, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.A.; Kiosses, W.B.; Alderson, N.B.; Meller, N.; Hahn, K.M.; Schwartz, M.A. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 2002, 4, 232. [Google Scholar] [CrossRef] [PubMed]
- Krauss, K.; Altevogt, P. Integrin Leukocyte Function-associated Antigen-1-mediated Cell Binding Can Be Activated by Clustering of Membrane Rafts. J. Biol. Chem. 1999, 274, 36921–36927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitinger, B.; Hogg, N. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 2002, 115, 963. [Google Scholar] [PubMed]
- Del Pozo, M.A.; Alderson, N.B.; Kiosses, W.B.; Chiang, H.-H.; Anderson, R.G.W.; Schwartz, M.A. Integrins regulate Rac targeting by internalization of membrane domains. Science 2004, 303, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia 2015, 29, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Kollet, O. The Brain-Bone-Blood Triad: Traffic Lights for Stem-Cell Homing and Mobilization. Hematology 2010, 2010, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bonig, H.; Papayannopoulou, T. Hematopoietic stem cell mobilization: Updated conceptual renditions. Leukemia 2013, 27, 24–31. [Google Scholar] [CrossRef]
- Greenbaum, A.M.; Link, D.C. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 2011, 25, 211–217. [Google Scholar] [CrossRef]
- Adamiak, M.; Poniewierska-Baran, A.; Borkowska, S.; Schneider, G.; Abdelbaset-Ismail, A.; Suszynska, M.; Abdel-Latif, A.; Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. Evidence that a lipolytic enzyme—Hematopoietic-specific phospholipase C-β2—promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes. Leukemia 2016, 30, 919–928. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Adamiak, M. Membrane Lipid Rafts, Master Regulators of Hematopoietic Stem Cell Retention in Bone Marrow, and Their Trafficking. Leukemia 2015, 29, 1452. [Google Scholar] [CrossRef] [PubMed]
- Shirvaikar, N.; Marquez-Curtis, L.A.; Shaw, A.R.; Turner, A.R.; Janowska-Wieczorek, A. MT1-MMP association with membrane lipid rafts facilitates G-CSF−induced hematopoietic stem/progenitor cell mobilization. Exp. Hematol. 2010, 38, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Shirvaikar, N.; Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem. Res. Int. 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Localizing matrix metalloproteinase activities in the pericellular environment. FEBS J. 2011, 278, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Orford, K.W.; Scadden, D.T. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Iwama, A.; Morita, Y.; Eto, K.; Ema, H.; Nakauchi, H. Cytokine Signaling, Lipid Raft Clustering, and HSC Hibernation. Ann. N. Y. Acad. Sci. 2007, 1106, 54–63. [Google Scholar] [CrossRef]
- Yamazaki, S.; Iwama, A.; Takayanagi, S.; Morita, Y.; Eto, K.; Ema, H.; Nakauchi, H. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006, 25, 3515–3523. [Google Scholar] [CrossRef] [Green Version]
- Seita, J.; Weissman, I.L. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef]
- Yamazaki, S.; Iwama, A.; Takayanagi, S.; Eto, K.; Ema, H.; Nakauchi, H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 2009, 113, 1250–1256. [Google Scholar] [CrossRef]
- Vannini, N.; Roch, A.; Naveiras, O.; Griffa, A.; Kobel, S.; Lutolf, M.P. Identification of in vitro HSC fate regulators by differential lipid raft clustering. Cell Cycle 2012, 11, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.; Leifheit, E.; Gooch, S.; Sindhu, S.; Weinberg, K. Lipid rafts are required for Kit survival and proliferation signals. Blood 2007, 110, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Wilsch-Bräuninger, M.; Karbanová, J.; Fonseca, A.-V.; Strauss, D.; Freund, D.; Thiele, C.; Huttner, W.B.; Bornhäuser, M.; Corbeil, D. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles—A role of the endocytic–exocytic pathway. EMBO Mol. Med. 2011, 3, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, A.; Corbeil, D.; Hellwig, A.; Huttner, W.B. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. USA 1997, 94, 12425–12430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a Novel Marker for Human Hematopoietic Stem and Progenitor Cells. Blood 1997, 90, 5002. [Google Scholar] [PubMed]
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A Novel Five-Transmembrane Hematopoietic Stem Cell Antigen: Isolation, Characterization, and Molecular Cloning. Blood 1997, 90, 5013. [Google Scholar]
- Bhatia, M. AC133 expression in human stem cells. Leukemia 2001, 15, 1685–1688. [Google Scholar] [CrossRef] [Green Version]
- Freund, D.; Bauer, N.; Boxberger, S.; Feldmann, S.; Streller, U.; Ehninger, G.; Werner, C.; Bornhäuser, M.; Oswald, J.; Corbeil, D. Polarization of Human Hematopoietic Progenitors During Contact with Multipotent Mesenchymal Stromal Cells: Effects on Proliferation and Clonogenicity. Stem Cells Dev. 2006, 15, 815–829. [Google Scholar] [CrossRef]
- Röper, K.; Corbeil, D.; Huttner, W.B. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2000, 2, 582–592. [Google Scholar] [CrossRef]
- Kuçi, S.; Wessels, J.T.; Bühring, H.-J.; Schilbach, K.; Schumm, M.; Seitz, G.; Löffler, J.; Bader, P.; Schlegel, P.G.; Niethammer, D.; et al. Identification of a novel class of human adherent CD34− stem cells that give rise to SCID-repopulating cells. Blood 2003, 101, 869–876. [Google Scholar] [CrossRef]
- Arndt, K.; Grinenko, T.; Mende, N.; Reichert, D.; Portz, M.; Ripich, T.; Carmeliet, P.; Corbeil, D.; Waskow, C. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 5582–5587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbanová, J.; Lorico, A.; Bornhäuser, M.; Corbeil, D.; Fargeas, C.A. Prominin--1/CD133: Lipid Raft Association, Detergent Resistance, and Immunodetection. Stem Cells Transl. Med. 2017, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.-M.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of Absorption and ABC1-Mediated Efflux of Cholesterol by RXR Heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Büchler, C.; Orsó, E.; Kaminski, W.E.; Porsch-Özcürümez, M.; Liebisch, G.; Kapinsky, M.; Diederich, W.; Drobnik, W.; Dean, M. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA 2000, 97, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, B.A.; Repa, J.J.; Joseph, S.B.; Wilpitz, D.C.; Kast, H.R.; Mangelsdorf, D.J.; Tontonoz, P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K.; Kritharides, L.; Schmitz, G.; Boettcher, A.; Drobnik, W.; Langmann, T.; Quinn, C.M.; Death, A.; Dean, R.T.; Jessup, W.; et al. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. FASEB J. 2004, 18, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.-L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Investig. 2011, 121, 4138. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Pagler, T.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; Snoeck, H.W.; et al. ATP-Binding Cassette Transporters and HDL Suppress Hematopoietic Stem Cell Proliferation. Science 2010, 328, 1689–1693. [Google Scholar] [CrossRef] [Green Version]
- Takehara, M.; Takagishi, T.; Seike, S.; Oishi, K.; Fujihara, Y.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens α-Toxin Impairs Lipid Raft Integrity in Neutrophils. Biol. Pharm. Bull. 2016, 39, 1694–1700. [Google Scholar] [CrossRef]
- Jiang, J.; Kao, C.-Y.; Papoutsakis, E.T. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J. Control. Release 2017, 247, 1–18. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomari, M.; Almohazey, D.; Almofty, S.A.; Khan, F.A.; Al hamad, M.; Ababneh, D. Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation. Cells 2019, 8, 630. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060630
Alomari M, Almohazey D, Almofty SA, Khan FA, Al hamad M, Ababneh D. Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation. Cells. 2019; 8(6):630. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060630
Chicago/Turabian StyleAlomari, Munther, Dana Almohazey, Sarah Ameen Almofty, Firdos Alam Khan, Mohammad Al hamad, and Deena Ababneh. 2019. "Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation" Cells 8, no. 6: 630. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8060630