Multiple Adenosine-Dopamine (A2A-D2 Like) Heteroreceptor Complexes in the Brain and Their Role in Schizophrenia
Abstract
:1. Introduction
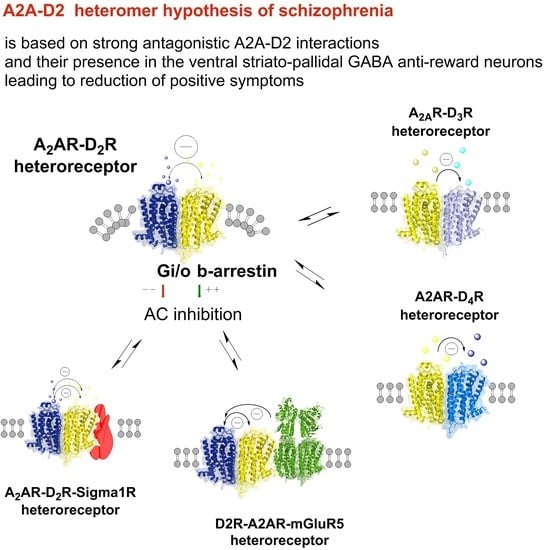
2. The A2A-D2 Receptor Heteromer Hypothesis and the LARA et al. Adenosine Hypothesis of Schizophrenia
3. A1-A2A Isoreceptor Complexes
4. A2A-A2B Isorecepor Complexes
5. A2A-D3 Heteroreceptor Complexes
6. A2A-D4 Heteroreceptor Complexes
7. A2AR-D2R-mGlu5R Heteroreceptor Complexes
8. A2A-D2-Sigma1 Heteroreceptor Complexes
9. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuxe, K.; Ferre, S.; Zoli, M.; Agnati, L.F. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine a2a/dopamine d2 and adenosine a1/dopamine d1 receptor interactions in the basal ganglia. Brain Res. Brain Res. Rev. 1998, 26, 258–273. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Guescini, M.; Fernandez-Duenas, V.; Tanganelli, S.; Rivera, A.; Ciruela, F.; Agnati, L.F. Adenosine-dopamine interactions in the pathophysiology and treatment of cns disorders. CNS Neurosci. Ther. 2010, 16, e18–e42. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Pintsuk, J.; Schafer, T.; Friedland, K.; Ferraro, L.; Tanganelli, S.; Liu, F.; Fuxe, K. Multiple d2 heteroreceptor complexes: New targets for treatment of schizophrenia. Ther. Adv. Psychopharmacol. 2016, 6, 77–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredholm, B.B. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol. 1995, 76, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; Fuxe, K. Adenosine as a volume transmission signal. A feedback detector of neuronal activation. Prog. Brain Res. 2000, 125, 353–361. [Google Scholar] [PubMed]
- Borroto-Escuela, D.O.; Agnati, L.F.; Bechter, K.; Jansson, A.; Tarakanov, A.O.; Fuxe, K. The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural-glial networks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciruela, F.; Casado, V.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. Immunological identification of a1 adenosine receptors in brain cortex. J. Neurosci. Res. 1995, 42, 818–828. [Google Scholar] [CrossRef]
- Zeraati, M.; Mirnajafi-Zadeh, J.; Fathollahi, Y.; Namvar, S.; Rezvani, M.E. Adenosine a1 and a2a receptors of hippocampal ca1 region have opposite effects on piriform cortex kindled seizures in rats. Seizure 2006, 15, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Schiffmann, S.N.; Fisone, G.; Moresco, R.; Cunha, R.A.; Ferre, S. Adenosine a2a receptors and basal ganglia physiology. Prog. Neurobiol. 2007, 83, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Schiffmann, S.N.; Vanderhaeghen, J.J. Adenosine a2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J. Neurosci. 1993, 13, 1080–1087. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. (Copenh) 1963, 20, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Anden, N.E.; Butcher, S.G.; Corrodi, H.; Fuxe, K.; Ungerstedt, U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Experientia 1970, 11, 303–314. [Google Scholar] [CrossRef]
- Seeman, P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987, 1, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Targeting the dopamine d2 receptor in schizophrenia. Expert Opin. Ther. Targets 2006, 10, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more d2 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeman, P.; Tallerico, T.; Ko, F.; Tenn, C.; Kapur, S. Amphetamine-sensitized animals show a marked increase in dopamine d2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse 2002, 46, 235–239. [Google Scholar] [CrossRef]
- Fuxe, K. Biological and pharmacological theories. Discussion. In The neuroleptics; Bobon, D.P., Janssen, P.A.J., Bobon, J., Eds.; Karger: Basel, Switzerland, 1970; pp. 121–122. [Google Scholar]
- Svensson, T.H. Dysfunctional brain dopamine systems induced by psychotomimetic nmda-receptor antagonists and the effects of antipsychotic drugs. Brain Res. Rev. 2000, 31, 320–329. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-Cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.; Langel, U.; Genedani, S.; Ferraro, L.; et al. Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 2008, 58, 415–452. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chu, X.P.; Mao, L.M.; Wang, M.; Lan, H.X.; Li, M.H.; Zhang, G.C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of d2r-nr2b interactions in response to cocaine. Neuron 2006, 52, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Ferre, S.; Von Euler, G.; Johansson, B.; Fredholm, B.B.; Fuxe, K. Stimulation of high-affinity adenosine a2 receptors decreases the affinity of dopamine d2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 7238–7241. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Wydra, K.; Filip, M.; Fuxe, K. A2ar-d2r heteroreceptor complexes in cocaine reward and addiction. Trends Pharmacol. Sci. 2018, 39, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Canals, M.; Marcellino, D.; Fanelli, F.; Ciruela, F.; De Benedetti, P.; Goldberg, S.R.; Neve, K.; Fuxe, K.; Agnati, L.F.; Woods, A.S.; et al. Adenosine a2a-dopamine d2 receptor-receptor heteromerization: Qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003, 278, 46741–46749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine a2a and dopamine d2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005, 26, 209–220. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Marcellino, D.; Narvaez, M.; Flajolet, M.; Heintz, N.; Agnati, L.; Ciruela, F.; Fuxe, K. A serine point mutation in the adenosine a2ar c-terminal tail reduces receptor heteromerization and allosteric modulation of the dopamine d2r. Biochem. Biophys. Res. Commun. 2010, 394, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Gomez-Soler, M.; Corrales, F.; Marcellino, D.; Narvaez, M.; Frankowska, M.; Flajolet, M.; Heintz, N.; et al. Characterization of the a2ar-d2r interface: Focus on the role of the c-terminal tail and the transmembrane helices. Biochem. Biophys. Res. Commun. 2010, 402, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Saitoh, O.; Yoshioka, K.; Nakata, H. Oligomerization of adenosine a2a and dopamine d2 receptors in living cells. Biochem. Biophys. Res. Commun. 2003, 306, 544–549. [Google Scholar] [CrossRef]
- Hillion, J.; Canals, M.; Torvinen, M.; Casado, V.; Scott, R.; Terasmaa, A.; Hansson, A.; Watson, S.; Olah, M.E.; Mallol, J.; et al. Coaggregation, cointernalization, and codesensitization of adenosine a2a receptors and dopamine d2 receptors. J. Biol. Chem. 2002, 277, 18091–18097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Ciruela, F.; Agnati, L.F.; Fuxe, K. On the existence of a possible a2a-d2-beta-arrestin2 complex: A2a agonist modulation of d2 agonist-induced beta-arrestin2 recruitment. J. Mol. Biol. 2011, 406, 687–699. [Google Scholar] [CrossRef]
- Rimondini, R.; Ferre, S.; Ogren, S.O.; Fuxe, K. Adenosine a2a agonists: A potential new type of atypical antipsychotic. Neuropsychopharmacology 1997, 17, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narvaez, M.; Wydra, K.; Tarakanov, A.O.; Li, X.; Millon, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the role of gpcr heteroreceptor complexes in modulating the brain networks in health and disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Ciruela, F.; Casado, V.; Rodrigues, R.J.; Lujan, R.; Burgueno, J.; Canals, M.; Borycz, J.; Rebola, N.; Goldberg, S.R.; Mallol, J.; et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine a1-a2a receptor heteromers. J. Neurosci. 2006, 26, 2080–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, S.; Navarro, G.; Borroto-Escuela, D.; Seibt, B.F.; Ammon, Y.C.; De Filippo, E.; Danish, A.; Lacher, S.K.; Cervinkova, B.; Rafehi, M.; et al. Adenosine a2a receptor ligand recognition and signaling is blocked by a2b receptors. Oncotarget 2018, 9, 13593–13611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristovao-Ferreira, S.; Navarro, G.; Brugarolas, M.; Perez-Capote, K.; Vaz, S.H.; Fattorini, G.; Conti, F.; Lluis, C.; Ribeiro, J.A.; McCormick, P.J.; et al. A1r-a2ar heteromers coupled to gs and g i/0 proteins modulate gaba transport into astrocytes. Purinergic Signal. 2013, 9, 433–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torvinen, M.; Marcellino, D.; Canals, M.; Agnati, L.F.; Lluis, C.; Franco, R.; Fuxe, K. Adenosine a2a receptor and dopamine d3 receptor interactions: Evidence of functional a2a/d3 heteromeric complexes. Mol. Pharmacol. 2005, 67, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Borroto-Escuela, D.O. Receptor-Receptor Interactions in the Central Nervous System; Humana Press: New York, NY, USA, 2018; Volume 140, p. 346. [Google Scholar]
- Cabello, N.; Gandia, J.; Bertarelli, D.C.; Watanabe, M.; Lluis, C.; Franco, R.; Ferre, S.; Lujan, R.; Ciruela, F. Metabotropic glutamate type 5, dopamine d2 and adenosine a2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009, 109, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Narvaez, M.; Wydra, K.; Pintsuk, J.; Pinton, L.; Jimenez-Beristain, A.; Di Palma, M.; Jastrzebska, J.; Filip, M.; Fuxe, K. Cocaine self-administration specifically increases a2ar-d2r and d2r-sigma1r heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol. Biochem. Behav. 2017, 155, 24–31. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Frankowska, M.; Ferraro, L.; Guidolin, D.; Ciruela, F.; Agnati, L.F. The changing world of g protein-coupled receptors: From monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J. Recept Signal. Transduct. Res. 2010, 30, 272–283. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Fuxe, K. Diversity and bias through dopamine d2r heteroreceptor complexes. Curr. Opin. Pharmacol. 2017, 32, 16–22. [Google Scholar] [CrossRef]
- Ferre, S.; O’Connor, W.T.; Snaprud, P.; Ungerstedt, U.; Fuxe, K. Antagonistic interaction between adenosine a2a receptors and dopamine d2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience 1994, 63, 765–773. [Google Scholar] [CrossRef]
- Popken, G.J.; Bunney, W.E.; Potkin, S.G.; Jones, E.G. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc. Natl. Acad. Sci. USA 2000, 97, 9276–9280. [Google Scholar] [CrossRef] [Green Version]
- Tanganelli, S.; Sandager Nielsen, K.; Ferraro, L.; Antonelli, T.; Kehr, J.; Franco, R.; Ferre, S.; Agnati, L.F.; Fuxe, K.; Scheel-Kruger, J. Striatal plasticity at the network level. Focus on adenosine a2a and d2 interactions in models of parkinson’s disease. Parkinsonism Relat Disord. 2004, 10, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, T.; Fuxe, K.; Agnati, L.; Mazzoni, E.; Tanganelli, S.; Tomasini, M.C.; Ferraro, L. Experimental studies and theoretical aspects on a2a/d2 receptor interactions in a model of parkinson’s disease. Relevance for l-dopa induced dyskinesias. J. Neurol. Sci. 2006, 248, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Ferre, S.; Lluis, C.; Franco, R.; Fuxe, K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal gaba neurons. Pharmacol. Rev. 2003, 55, 509–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Pei, L.; Fletcher, P.J.; Kapur, S.; Seeman, P.; Liu, F. Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: Increased dopamine d2 receptor dimerization. Mol. Brain 2010, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Pintsuk, J.; Borroto-Escuela, D.O.; Lai, T.K.; Liu, F.; Fuxe, K. Alterations in ventral and dorsal striatal allosteric a2ar-d2r receptor-receptor interactions after amphetamine challenge: Relevance for schizophrenia. Life Sci. 2016, 167, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kurumaji, A.; Toru, M. An increase in [3h] cgs21680 binding in the striatum of postmortem brains of chronic schizophrenics. Brain Res. 1998, 808, 320–323. [Google Scholar] [CrossRef]
- Grace, A.A. Dopamine system dysregulation and the pathophysiology of schizophrenia: Insights from the methylazoxymethanol acetate model. Boil. Psychiatry 2015, 81, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Lara, D.R.; Dall’Igna, O.P.; Ghisolfi, E.S.; Brunstein, M.G. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2006, 30, 617–629. [Google Scholar] [CrossRef]
- Lara, D.R.; Brunstein, M.G.; Ghisolfi, E.S.; Lobato, M.I.; Belmonte-de-Abreu, P.; Souza, D.O. Allopurinol augmentation for poorly responsive schizophrenia. Int. Clin. Psychopharmacol. 2001, 16, 235–237. [Google Scholar] [CrossRef]
- Lara, D.R.; Vianna, M.R.M.; De Paris, F.; Quevedo, J.; Oses, J.P.; Battastini, A.M.O.; Sarkis, J.J.F.; Souza, D.O. Chronic treatment with clozapine, but not haloperidol, increases striatal ecto-5′-nucleotidase activity in rats. Neuropsychobiology 2001, 44, 99–102. [Google Scholar] [CrossRef]
- Lara, D.R.; Souza, D.O. Schizophrenia: A purinergic hypothesis. Med. Hypotheses 2000, 54, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Singer, P.; Shen, H.Y.; Feldon, J.; Yee, B.K. Adenosine hypothesis of schizophrenia—Opportunities for pharmacotherapy. Neuropharmacology 2012, 62, 1527–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Hagman, B.; Woolfenden, M.; Pinton, L.; Jiménez-Beristain, A.; Oflijan, J.; Narvaez, M.; Di Palma, M.; Feltmann, K.; Sartini, S.; et al. In situ proximity ligation assay to study and understand the distribution and balance of gpcr homo- and heteroreceptor complexes in the brain. In Receptor and Ion Channel Detection in the Brain; Lujan, R., Ciruela, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 110, pp. 109–126. [Google Scholar]
- Borroto-Escuela, D.O.; Brito, I.; Di Palma, M.; Jiménez-Beristain, A.; Narvaez, M.; Corrales, F.; Pita-Rodríguez, M.; Sartini, S.; Ambrogini, P.; Lattanzi, D.; et al. On the role of the balance of gpcr homo/ heteroreceptor complexes in the brain. J. Adv. Neurosci. Res. 2015, 2, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.O.; Tarakanov, A.O.; Romero-Fernandez, W.; Ferraro, L.; Tanganelli, S.; Perez-Alea, M.; Di Palma, M.; Agnati, L.F. Dopamine d2 heteroreceptor complexes and their receptor-receptor interactions in ventral striatum: Novel targets for antipsychotic drugs. Prog. Brain Res. 2014, 211, 113–139. [Google Scholar]
- Kostrzewa, R.M.; Wydra, K.; Filip, M.; Crawford, C.A.; McDougall, S.A.; Brown, R.W.; Borroto-Escuela, D.O.; Fuxe, K.; Gainetdinov, R.R. Dopamine d2 receptor supersensitivity as a spectrum of neurotoxicity and status in psychiatric disorders. J. Pharmacol. Exp. Ther. 2018, 366, 519–526. [Google Scholar] [CrossRef]
- Sokoloff, P.; Le Foll, B. The dopamine d3 receptor, a quarter century later. Eur. J. Neurosci. 2017, 45, 2–19. [Google Scholar] [CrossRef]
- Bristow, L.J.; Collinson, N.; Cook, G.P.; Curtis, N.; Freedman, S.B.; Kulagowski, J.J.; Leeson, P.D.; Patel, S.; Ragan, C.I.; Ridgill, M.; et al. L-745,870, a subtype selective dopamine d4 receptor antagonist, does not exhibit a neuroleptic-like profile in rodent behavioral tests. J. Pharmacol. Exp. Ther. 1997, 283, 1256–1263. [Google Scholar]
- Tarazi, F.I.; Zhang, K.; Baldessarini, R.J. Dopamine d4 receptors: Beyond schizophrenia. J. Recept. Signal. Transduct. Res. 2004, 24, 131–147. [Google Scholar] [CrossRef]
- Furth, K.E.; Mastwal, S.; Wang, K.H.; Buonanno, A.; Vullhorst, D. Dopamine, cognitive function, and gamma oscillations: Role of d4 receptors. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Ferre, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueno, J.; Gutierrez, M.A.; Casado, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic interaction between adenosine a2a and glutamate mglu5 receptors: Implications for striatal neuronal function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Agnati, L.F.; Jacobsen, K.; Hillion, J.; Canals, M.; Torvinen, M.; Tinner-Staines, B.; Staines, W.; Rosin, D.; Terasmaa, A.; et al. Receptor heteromerization in adenosine a2a receptor signaling: Relevance for striatal function and parkinson’s disease. Neurology 2003, 61, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Popoli, P.; Pezzola, A.; Torvinen, M.; Reggio, R.; Pintor, A.; Scarchilli, L.; Fuxe, K.; Ferre, S. The selective mglu(5) receptor agonist chpg inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine d(2) receptors in the rat striatum: Interactions with adenosine a(2a) receptors. Neuropsychopharmacology 2001, 25, 505–513. [Google Scholar] [CrossRef]
- Wieronska, J.M.; Zorn, S.H.; Doller, D.; Pilc, A. Metabotropic glutamate receptors as targets for new antipsychotic drugs. Pharmacol. Ther. 2016, 157, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Beggiato, S.; Borelli, A.C.; Borroto-Escuela, D.; Corbucci, I.; Tomasini, M.C.; Marti, M.; Antonelli, T.; Tanganelli, S.; Fuxe, K.; Ferraro, L. Cocaine modulates allosteric d2-sigma1 receptor-receptor interactions on dopamine and glutamate nerve terminals from rat striatum. Cell. Signal. 2017, 40, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farre, D.; Aguinaga, D.; Mallol, J.; Cortes, A.; Casado, V.; Lluis, C.; et al. Cocaine inhibits dopamine d2 receptor signaling via sigma-1-d2 receptor heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, L.; Borroto-Escuela, D.O.; Narváez, M.; Jiménez-Beristain, A.; Oflijan, J.; Ferraro, L.; Agnati, L.F.; Fuxe, K. Dopamine d2 receptor dynamic and modulation in the d2r-sigma1r heteroreceptor complexes: Role in cocaine actions. In European Neuropsychopharmacology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 25, pp. S609–S610. [Google Scholar]
- Pinton, L.; Borroto-Escuela, D.O.; Narváez, M.; Oflijan, J.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine d2r and sigma 1 allosteric receptor-receptor interaction in the rat brain: Role in brain plasticity and cocaine action. In European Society of Neurochemistry; Springer: Berlin/Heidelberg, Germany, 2015; Volume 4, p. P37. [Google Scholar]
- Kourrich, S.; Su, T.P.; Fujimoto, M.; Bonci, A. The sigma-1 receptor: Roles in neuronal plasticity and disease. Trends Neurosci. 2012, 35, 762–771. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borroto-Escuela, D.O.; Ferraro, L.; Narvaez, M.; Tanganelli, S.; Beggiato, S.; Liu, F.; Rivera, A.; Fuxe, K. Multiple Adenosine-Dopamine (A2A-D2 Like) Heteroreceptor Complexes in the Brain and Their Role in Schizophrenia. Cells 2020, 9, 1077. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9051077
Borroto-Escuela DO, Ferraro L, Narvaez M, Tanganelli S, Beggiato S, Liu F, Rivera A, Fuxe K. Multiple Adenosine-Dopamine (A2A-D2 Like) Heteroreceptor Complexes in the Brain and Their Role in Schizophrenia. Cells. 2020; 9(5):1077. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9051077
Chicago/Turabian StyleBorroto-Escuela, Dasiel O., Luca Ferraro, Manuel Narvaez, Sergio Tanganelli, Sarah Beggiato, Fang Liu, Alicia Rivera, and Kjell Fuxe. 2020. "Multiple Adenosine-Dopamine (A2A-D2 Like) Heteroreceptor Complexes in the Brain and Their Role in Schizophrenia" Cells 9, no. 5: 1077. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9051077