Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review
Abstract
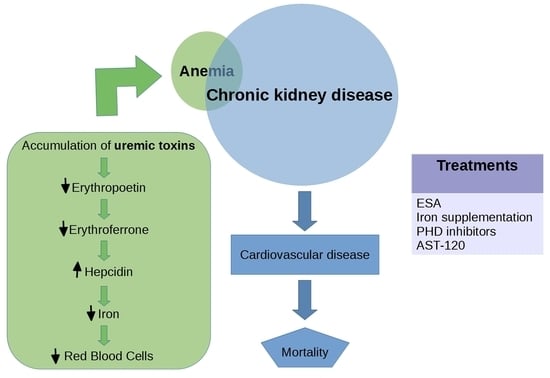
:1. Introduction
2. Uremic Toxins Are Central Players of the CKD Clinical Course
2.1. Representative Uremic Toxins: Indoxyl Sulfate and P-Cresyl Sulfate
2.2. IS Effects in Renal Anemia
2.3. Other Uremic Toxins Implicated in Erythropoiesis
3. Physiopathology of Anemia in CKD
3.1. Regulation of HIF in Kidney Disease
3.2. Impairment of IS-Induced HIF Activation
3.3. EPO and Regulation of Erythropoeisis
3.4. The Role of Iron in the Regulation of Erythropoeisis
3.5. Hepcidin and Regulation of Serum Iron Levels
4. Current Strategies of Renal Anemia Treatment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stam, F.; Van Guldener, C.; Schalkwijk, C.G.; Ter Wee, P.M.; Donker, A.J.M.; Stehouwer, C.D.A. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol. Dial. Transplant. 2003, 18, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.C.; Glorieux, G.; De Smet, R.; Lameire, N. New insights in uremic toxins. Kidney Int. 2003, 63, S6–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A Bench to Bedside View of Uremic Toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, A.; Tetta, C.; Ersoy, F.F.; D’Intini, V.; Ratanarat, R.; De Cal, M.; Bonello, M.; Bordoni, V.; Salvatori, G.; Andrikos, E.; et al. Reviews: Uremic Toxins: A New Focus on an Old Subject. Semin. Dial. 2005, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argilés, À. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. Int. Urol. Nephrol. 2012, 45, 139–150. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B. Uremic Toxins and Their Effects on Multiple Organ Systems. Nephron Clin. Pr. 2014, 128, 303–311. [Google Scholar] [CrossRef]
- MacDougall, I.C. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int. 2001, 59, S67–S72. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, P.J. Molecular biology of erythropoietin. Kidney Int. 1993, 44, 887–904. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-Y.; Mcculloch, C.; Curhan, G.C. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J. Am. Soc. Nephrol. 2002, 13, 7. [Google Scholar]
- Nangaku, M.; Eckardt, K.-U. Pathogenesis of Renal Anemia. Semin. Nephrol. 2006, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of Kidney Function With Anemia. Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V. The 3 Most Common Causes of Chronic Kidney Disease, Verywell Health, Posted July 24, 2019. Available online: https://www.verywellhealth.com/common-causes-of-chronic-kidney-disease-2085786 (accessed on 22 February 2020).
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic Kidney Disease and Its Complications. Prim. Care: Clin. Off. Pr. 2008, 35, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Khanna, A.; White, W.B. The Management of Hyperkalemia in Patients with Cardiovascular Disease. Am. J. Med. 2009, 122, 215–221. [Google Scholar] [CrossRef] [PubMed]
- NKF-K, I.V. Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: Update. Am. J. Kidney Dis. 2001, 37, S182–S238. [Google Scholar] [CrossRef]
- WHO Scientific Group. Nutritional Anemias; Technical Report Series No. 405; World Health Organization: Geneva, Switzerland, 1968; Available online: https://apps.who.int/iris/bitstream/handle/10665/40707/WHO_TRS_405.pdf (accessed on 27 March 2020).
- McClellan, W.M.; Aronoff, S.L.; Bolton, W.K.; Hood, S.; Lorber, D.L.; Tang, K.L.; Tse, T.F.; Wasserman, B.; Leiserowitz, M. The prevalence of anemia in patients with chronic kidney disease. Curr. Med Res. Opin. 2004, 20, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V. Epidemiology of Anemia in Older Adults. Semin. Hematol. 2008, 45, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Weiner, D.E.; Tighiouart, H.; Vlagopoulos, P.T.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Effects of Anemia and Left Ventricular Hypertrophy on Cardiovascular Disease in Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2005, 16, 1803–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Hypertens. 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Cases, A.; Coll, E.; Collado, S. Anemia en la insuficiencia renal crónica y sus implicaciones cardiovasculares. Med. Clínica 2009, 132, 38–42. [Google Scholar] [CrossRef]
- Agarwal, A.K. Practical Approach to the Diagnosis and Treatment of Anemia Associated With CKD in Elderly. J. Am. Med. Dir. Assoc. 2006, 7, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Wagner, Z.; Molnár, M.; Molnár, G.A.; Tamaskó, M.; Laczy, B.; Wagner, L.; Csiky, B.; Heidland, A.; Nagy, J.; Wittmann, I. Serum Carboxymethyllysine Predicts Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2006, 47, 294–300. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2009, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum β-2 Microglobulin Levels Predict Mortality in Dialysis Patients: Results of the HEMO Study. J. Am. Soc. Nephrol. 2005, 17, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.C.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Wengle, B.; Hellström, K. Volatile Phenols in Serum of Uraemic Patients. Clin. Sci. 1972, 43, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar] [CrossRef]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the Accumulation of Uremic Toxins in the Blood and Their Precursors in the Feces after Oral Administration of Lebenin®, a Lactic Acid Bacteria Preparation, to Uremic Patients Undergoing Hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef]
- Kabanda, A.; Jadoul, M.; Pochet, J.M.; Lauwerys, R.; Strihou, C.V.Y.D.; Bernard, A. Determinants of the serum concentrations of low molecular weight proteins in patients on maintenance hemodialysis. Kidney Int. 1994, 45, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, G.; Franke, S.; Mahiout, A.; Schneider, S.; Sperschneider, H.; Borst, S.; Vienken, J. Influence of dialysis modalities on serum AGE levels in end-stage renal disease patients. Nephrol. Dial. Transplant. 2001, 16, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.C.; Ringoir, S.M. Adequacy of dialysis: A critical analysis. Kidney Int. 1992, 42, 540–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, W.F.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The Urea Reduction Ratio and Serum Albumin Concentration as Predictors of Mortality in Patients Undergoing Hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar] [CrossRef]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58, S47–S59. [Google Scholar] [CrossRef] [Green Version]
- Lesaffer, G.; De Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R.C. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef]
- Liabeuf, S.; Drüeke, T.B.; Massy, Z.A. Protein-Bound Uremic Toxins: New Insight from Clinical Studies. Toxins 2011, 3, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-K.; Tanaka, T.; Inagi, R.; Fujita, T.; Nangaku, M. Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab. Investig. 2011, 91, 1564–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.-K.; Tanaka, T.; Nangaku, M. Dysregulated Oxygen Metabolism of the Kidney by Uremic Toxins: Review. J. Ren. Nutr. 2012, 22, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 2010, 72, 1–11. [Google Scholar]
- Vanholder, R.; Smet, R.D. Pathophysiologic Effects of Uremic Retention Solutes. JASN 1999, 10, 1815–1823. [Google Scholar]
- Vanholder, R.; Bammens, B.; De Loor, H.; Glorieux, G.; Meijers, B.; Schepers, E.; Massy, Z.; Evenepoel, P. Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol. Dial. Transplant. 2011, 26, 1464–1467. [Google Scholar] [CrossRef] [Green Version]
- Viaene, L.; Annaert, P.; Loor, H.D.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin is the main plasma binding protein for indoxyl sulfate and p -cresyl sulfate: Albumin is main carrier of indoxyl sulfate and p -cresyl sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chen, H.-H.; Pan, C.-F.; Chuang, C.-K.; Wang, T.-J.; Sun, F.-J.; Wu, C.-J. p-cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011, 25, 191–197. [Google Scholar] [CrossRef]
- Niwa, T.; Ise, M.; Miyazaki, T. Progression of Glomerular Sclerosis in Experimental Uremic Rats by Administration of Indole, a Precursor of Indoxyl Sulfate. Am. J. Nephrol. 1994, 14, 207–212. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic Toxins Induce Kidney Fibrosis by Activating Intrarenal Renin–Angiotensin–Aldosterone System Associated Epithelial-to-Mesenchymal Transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.-W.; Hsu, K.-H.; Lee, C.-C.; Sun, C.-Y.; Hsu, H.-J.; Tsai, C.-J.; Tzen, C.-Y.; Wang, Y.-C.; Lin, C.-Y.; Wu, M.-S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 26, 938–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-J.; Liu, H.-L.; Pan, C.-F.; Chuang, C.-K.; Jayakumar, T.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. Indoxyl Sulfate Predicts Cardiovascular Disease and Renal Function Deterioration in Advanced Chronic Kidney Disease. Arch. Med. Res. 2012, 43, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, T.S.; Poitevin, S.; Paul, P.; McKay, N.; Jourde-Chiche, N.; Legris, T.; Mouly-Bandini, A.; Dignat-George, F.; Brunet, P.; Masereeuw, R.; et al. Indoxyl Sulfate Upregulates Liver P-Glycoprotein Expression and Activity through Aryl Hydrocarbon Receptor Signaling. J. Am. Soc. Nephrol. 2018, 29, 906–918. [Google Scholar]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The Uremic Toxicity of Indoxyl Sulfate and p-Cresyl Sulfate: A Systematic Review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Taki, K.; Tsuruta, Y.; Niwa, T. Indoxyl Sulfate and Atherosclerotic Risk Factors in Hemodialysis Patients. Am. J. Nephrol. 2007, 27, 30–35. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; on behalf of the European Uremic Toxin Work Group (EUTox). Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-κB and free radical in proximal tubular cells. Kidney Int. 2003, 63, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Hear. J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-J.; Pan, C.-F.; Liu, H.-L.; Chuang, C.-K.; Jayakumar, T.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atheroscler. 2012, 225, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-A.; Lu, L.-F.; Yu, T.-H.; Hung, W.-C.; Chung, F.-M.; Tsai, I.-T.; Yang, C.-Y.; Hsu, C.-C.; Lu, Y.-C.; Wang, C.-P.; et al. Increased Levels of Total P-Cresylsulphate and Indoxyl Sulphate are Associated with Coronary Artery Disease in Patients with Diabetic Nephropathy. Rev. Diabet. Stud. 2011, 7, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.S.E.; Abed, M.; Voelkl, J.; Lang, F. Triggering of suicidal erythrocyte death by uremic toxin indoxyl sulfate. Bmc Nephrol. 2013, 14, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelibieke, Y.; Shimizu, H.; Saito, S.; Mironova, R.; Niwa, T. Indoxyl sulfate counteracts endothelial effects of erythropoietin through suppression of Akt phosphorylation. Circ. J. 2013, 77, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- Congote, L.F.; Sadvakassova, G.; Dobocan, M.C.; Difalco, M.R.; Li, Q. Erythropoietin-dependent endothelial proteins: Potential use against erythropoietin resistance. Cytokine 2010, 51, 113–118. [Google Scholar] [CrossRef]
- Congote, L.F.; Difalco, M.R.; Gibbs, B.F. Thrombospondin 1, produced by endothelial cells under the action of erythropoietin, stimulates thymidine incorporation into erythroid cells and counteracts the inhibitory action of insulin-like growth factor binding protein. Cytokine 2005, 30, 248–253. [Google Scholar] [CrossRef]
- Asai, H.; Hirata, J.; Hirano, A.; Hirai, K.; Seki, S.; Watanabe-Akanuma, M. Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulfate. Am. J. Physiol. Physiol. 2016, 310, C142–C150. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Ji, S.; Dong, W.; Qi, Y.; Song, W.; Cui, D.; Shi, J. Indolic Uremic Solutes Enhance Procoagulant Activity of Red Blood Cells through Phosphatidylserine Exposure and Microparticle Release. Toxins 2015, 7, 4390–4403. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Meng, X.W.; Flatten, K.S.; Loegering, D.K.S.; Kaufmann, S.H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2012, 20, 64–76. [Google Scholar] [CrossRef]
- Wu, C.-J.; Chen, C.-Y.; Lai, T.-S.; Wu, P.-C.; Chuang, C.-K.; Sun, F.-J.; Liu, H.-L.; Chen, H.-H.; Yeh, H.-I.; Lin, C.-S.; et al. Correction: The role of indoxyl sulfate in renal anemia in patients with chronic kidney disease. Oncotarget 2019, 10, 2006. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Pelletier, M.; Sallée, M.; Berland, Y.; McKay, N.; Duval, A.; Gentile, S.; Mouelhi, Y.; Brunet, P.; Burtey, S. Indole 3-acetic acid, indoxyl sulfate and paracresyl-sulfate do not influence anemia parameters in hemodialysis patients. Bmc Nephrol. 2017, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.F.; Bonan, N.B.; Steiner, T.M.; Tozoni, S.S.; Rodrigues, S.D.; Nakao, L.S.; Kuntsevich, V.; Pecoits-Filho, R.; Kotanko, P.; Moreno-Amaral, A.N. Indoxyl Sulfate, a Uremic Toxin, Stimulates Reactive Oxygen Species Production and Erythrocyte Cell Death Supposedly by an Organic Anion Transporter 2 (OAT2) and NADPH Oxidase Activity-Dependent Pathways. Toxins 2018, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Yoneda, T.; Kimura, S.; Fujimoto, K.; Okajima, E.; Hirao, Y. Polyamines as an Inhibitor on Erythropoiesis of Hemodialysis Patients by In Vitro Bioassay Using the Fetal Mouse Liver Assay. Ther. Apher. Dial. 2006, 10, 267–272. [Google Scholar] [CrossRef]
- Kushner, D.; Beckman, B.; Nguyen, L.; Chen, S.; Della Santina, C.; Husserl, F.; Rice, J.; Fisher, J.W. Polyamines in the anemia of end-stage renal disease. Kidney Int. 1991, 39, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Radtke, H.W.; Rege, A.B.; Lamarche, M.B.; Bartos, D.; Bartos, F.; Campbell, R.A.; Fisher, J.W. Identification of Spermine as an Inhibitor of Erythropoiesis in Patients with Chronic Renal Failure. J. Clin. Investig. 1981, 67, 1623–1629. [Google Scholar] [CrossRef]
- Ahmed, M.S.E.; Langer, H.; Abed, M.; Voelkl, J.; Lang, F. The Uremic Toxin Acrolein Promotes Suicidal Erythrocyte Death. Kidney Blood Press. Res. 2013, 37, 158–167. [Google Scholar] [CrossRef]
- Freedman, M.H.; Cattrarn, D.C.; Saunders, F. Anemia of Chronic Renal Failure: Inhibition of Erythropoiesis by Uremic Serum. Nephron 1983, 35, 15–19. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and Characterization of Hypoxia-inducible Factor. J. Boil. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, R.; Sutherland, S.; Van Wesel, A.C.; Huizinga, E.G.; Percy, M.J.; Bierings, M.; Lee, F.S. Erythrocytosis associated with a novel missense mutation in the HIF2A gene. Haematol. 2009, 95, 829–832. [Google Scholar] [CrossRef] [Green Version]
- Percy, M.J.; Furlow, P.W.; Lucas, G.S.; Li, X.; Lappin, T.R.; McMullin, M.F.; Lee, F.S. A Gain-of-Function Mutation in theHIF2AGene in Familial Erythrocytosis. N. Engl. J. Med. 2008, 358, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scortegagna, M.; Ding, K.; Zhang, Q.; Oktay, Y.; Bennett, M.J.; Shelton, J.M.; Richardson, J.A.; Moe, O.; Garcia, J.A.; Bennett, M. HIF-2α regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 2005, 105, 3133–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliege, A.; Rosenberger, C.; Bondke, A.; Sciesielski, L.K.; Shina, A.; Heyman, S.N.; Flippin, L.A.; Arend, M.; Klaus, S.J.; Bachmann, S. Hypoxia-inducible factor-2α-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010, 77, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, V.H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 2017, 21, S110–S124. [Google Scholar] [CrossRef] [Green Version]
- Koury, M.J.; Haase, V.H. Anaemia in kidney disease: Harnessing hypoxia responses for therapy. Nat. Rev. Nephrol. 2015, 11, 394–410. [Google Scholar] [CrossRef] [Green Version]
- Mimura, I.; Nangaku, M. The suffocating kidney: Tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 2010, 6, 667–678. [Google Scholar] [CrossRef]
- Tanaka, T.; Nangaku, M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010, 19, 43–50. [Google Scholar] [CrossRef]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Bernhardt, W.M.; Wiesener, M.S.; Scigalla, P.; Chou, J.; Schmieder, R.E.; Günzler, V.; Eckardt, K.-U. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J. Am. Soc. Nephrol. 2010, 21, 2151–2156. [Google Scholar] [CrossRef]
- Franke, K.; Kalucka, J.; Mamlouk, S.; Singh, R.P.; Muschter, A.; Weidemann, A.; Iyengar, V.; Jahn, S.; Wieczorek, K.; Geiger, K.; et al. HIF-1α is a protective factor in conditional PHD2-deficient mice suffering from severe HIF-2α–induced excessive erythropoiesis. Blood 2013, 121, 1436–1445. [Google Scholar] [CrossRef]
- Scortegagna, M.; Morris, M.A.; Oktay, Y.; Bennett, M.; Garcia, J.A. The HIF family member EPAS1/HIF-2α is required for normal hematopoiesis in mice. Blood 2003, 102, 1634–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapitsinou, P.P.; Liu, Q.; Unger, T.L.; Rha, J.; Davidoff, O.; Keith, B.; Epstein, J.A.; Moores, S.L.; Erickson-Miller, C.L.; Haase, V.H. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 2010, 116, 3039–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Yamaguchi, J.; Higashijima, Y.; Nangaku, M. Indoxyl sulfate signals for rapid mRNA stabilization of Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) and suppresses the expression of hypoxia-inducible genes in experimental CKD and uremia. Faseb J. 2013, 27, 4059–4075. [Google Scholar] [CrossRef] [PubMed]
- Testa, U. Apoptotic mechanisms in the control of erythropoiesis. Leuk. 2004, 18, 1176–1199. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.W. Erythropoietin: Physiology and Pharmacology Update. Exp. Boil. Med. 2003, 228, 1–14. [Google Scholar] [CrossRef]
- Lin, C.S.; Lim, S.K.; D’Agati, V.; Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996, 10, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.P.; Fang, J.; Wojchowski, D.M. Core erythropoietin receptor signals for late erythroblast development. Blood 2006, 107, 2662–2672. [Google Scholar] [CrossRef]
- Richmond, T.D.; Chohan, M.; Barber, D.L. Turning cells red: Signal transduction mediated by erythropoietin. Trends Cell Boil. 2005, 15, 146–155. [Google Scholar] [CrossRef]
- Wojchowski, D.M.; Gregory, R.C.; Miller, C.P.; Pandit, A.K.; Pircher, T.J. Signal Transduction in the Erythropoietin Receptor System. Exp. Cell Res. 1999, 253, 143–156. [Google Scholar] [CrossRef]
- Jelkmann, W. Erythropoietin after a century of research: Younger than ever. Eur. J. Haematol. 2007, 78, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Subramaniam, V.N. The relationship between systemic iron homeostasis and erythropoiesis. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.; Nemeth, E. New insights into iron regulation and erythropoiesis. Curr. Opin. Hematol. 2015, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Gulbins, E.; Lang, P.A.; Zappulla, D.; Föller, M. Ceramide in Suicidal Death of Erythrocytes. Cell. Physiol. Biochem. 2010, 26, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J. Boil. Chem. 2000, 276, 7806–7810. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Molecular Control of Iron Transport. J. Am. Soc. Nephrol. 2007, 18, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T.; Nemeth, E. Hepcidin and Disorders of Iron Metabolism. Annu. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; Ishikawa, I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood 2006, 108, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Troutt, J.S.; Butterfield, A.M.; Konrad, R.J. Hepcidin-25 Concentrations Are Markedly Increased in Patients With Chronic Kidney Disease and Are Inversely Correlated With Estimated Glomerular Filtration Rates. J. Clin. Lab. Anal. 2013, 27, 504–510. [Google Scholar] [CrossRef]
- Singh, A.K. Erythropoiesis. In Textbook of Nephro-Endocrinology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 207–215. [Google Scholar]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Paulson, R.F.; Shi, L.; Wu, D.-C. Stress erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, R.; Ganz, T. Erythroferrone: An Erythroid Regulator of Hepcidin and Iron Metabolism. HemaSphere 2018, 2, e35. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Ikeda, Y.; Watanabe, H.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Imanishi, M.; Zamami, Y.; Takechi, K.; Miyamoto, L.; Ishizawa, K.; et al. The uremic toxin indoxyl sulfate interferes with iron metabolism by regulating hepcidin in chronic kidney disease. Nephrol. Dial. Transplant. 2017, 33, 586–597. [Google Scholar] [CrossRef]
- Coyne, D.W. Use of Epoetin in Chronic Renal Failure. JAMA 2007, 297, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, J.W.; Egrie, J.C.; Downing, M.R.; Browne, J.K.; Adamson, J.W. Correction of the Anemia of End-Stage Renal Disease with Recombinant Human Erythropoietin. N. Engl. J. Med. 1987, 316, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, J.W.; Abdulhadi, M.H.; Browne, J.K.; Delano, B.G.; Downing, M.R.; Egrie, J.C.; Evans, R.W.; Friedman, E.A.; Graber, S.E.; Haley, N.R.; et al. Recombinant Human Erythropoietin in Anemic Patients with End-Stage Renal Disease: Results of a Phase III Multicenter Clinical Trial. Ann. Intern. Med. 1989, 111, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Rocco, M. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease: 2007 Update of Hemoglobin Target. Am. J. Kidney Dis. 2007, 50, 471–530. [Google Scholar] [CrossRef]
- McMurray, J.J.; Parfrey, P.S.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Supp. 2012, 64. [Google Scholar]
- Drüeke, T.B.; Locatelli, F.; Clyne, N.; Eckardt, K.-U.; MacDougall, I.C.; Tsakiris, D.; Burger, H.-U.; Scherhag, A. Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia. N. Engl. J. Med. 2006, 355, 2071–2084. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Szczech, L.; Tang, K.L.; Barnhart, H.; Sapp, S.; Wolfson, M.; Reddan, N. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N. Engl. J. Med. 2006, 355, 2085–2098. [Google Scholar] [CrossRef] [Green Version]
- Fishbane, S. Upper Limit of Serum Ferritin: Misinterpretation of the 2006 KDOQI Anemia Guidelines: Upper limit of serum ferritin: Misinterpretation of guidelines. Semin. Dial. 2008, 21, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Lee, G.H.; Miller, J.E.; Streja, E.; Jing, J.; Robertson, J.A.; Kovesdy, C.P. Predictors of Hyporesponsiveness to Erythropoiesis-Stimulating Agents in Hemodialysis Patients. Am. J. Kidney Dis. 2009, 53, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besarab, A.; Coyne, D.W. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 699–710. [Google Scholar] [CrossRef]
- Besarab, A.; Kaiser, J.W.; Frinak, S. A study of parenteral iron regimens in hemodialysis patients. Am. J. Kidney Dis. 1999, 34, 21–28. [Google Scholar] [CrossRef]
- Besarab, A.; Amin, N.; Ahsan, M.; Vogel, S.E.; Zazuwa, G.; Frinak, S.; Zazra, J.J.; Anandan, J.V.; Gupta, A.P. Optimization of Epoetin Therapy with Intravenous Iron Therapy in Hemodialysis Patients. J. Am. Soc. Nephrol. 2000, 11, 530–538. [Google Scholar]
- Hsieh, M.M.; Linde, N.S.; Wynter, A.; Metzger, M.; Wong, C.; Langsetmo, I.; Lin, A.; Smith, R.; Rodgers, G.P.; Donahue, R.E.; et al. HIF–prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood 2007, 110, 2140–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDougall, I.C.; Eckardt, K.-U. Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet 2006, 368, 947–953. [Google Scholar] [CrossRef]
- Chen, N.; Hao, C.; Peng, X.; Lin, H.; Yin, A.; Hao, L.; Tao, Y.; Liang, X.; Liu, Z.; Xing, C.; et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N. Engl. J. Med. 2019, 381, 1001–1010. [Google Scholar] [CrossRef]
- Sugahara, M.; Tanaka, T.; Nangaku, M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 2017, 92, 306–312. [Google Scholar] [CrossRef]
- Niwa, T.; Yazawa, T.; Ise, M.; Sugano, M.; Kodama, T.; Uehara, Y.; Maeda, K. Inhibitory Effect of Oral Sorbent on Accumulation of Albumin-Bound Indoxyl Sulfate in Serum of Experimental Uremic Rats. Nephron 1991, 57, 84–88. [Google Scholar] [CrossRef]
- Niwa, T.; Emoto, Y.; Maeda, K.; Uehara, Y.; Yamada, N.; Shibata, M.; Nobuo, Y. Oral Sorbent Suppresses Accumulation of Albumin-Bound Indoxyl Sulphate in Serum of Haemodialysis Patients. Nephrol. Dial. Transplant. 1991, 6, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Miyazaki, T.; Hashimoto, N.; Hayashi, H.; Ise, M.; Uehara, Y.; Maeda, K. Suppressed Serum and Urine Levels of Indoxyl Sulfate by Oral Sorbent in Experimental Uremic Rats. Am. J. Nephrol. 1992, 12, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Aoyama, I.; Ise, M.; Seo, H.; Niwa, T. An oral sorbent reduces overload of indoxyl sulphate and gene expression of TGF-β1 in uraemic rat kidneys. Nephrol. Dial. Transplant. 2000, 15, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, I.-W.; Hsu, K.-H.; Sun, C.-Y.; Tsai, C.-J.; Wu, M.-S.; Lee, C.-C. Oral adsorbent AST-120 potentiates the effect of erythropoietin-stimulating agents on Stage 5 chronic kidney disease patients: A randomized crossover study. Nephrol. Dial. Transplant. 2014, 29, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.D. Practical considerations for iron therapy in the management of anaemia in patients with chronic kidney disease. Clin. Kidney J. 2017, 10, i9–i15. [Google Scholar] [CrossRef]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Hao, C.; Liu, B.-C.; Lin, H.; Wang, C.; Xing, C.; Liang, X.; Jiang, G.; Liu, Z.; Li, X.; et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N. Engl. J. Med. 2019, 381, 1011–1022. [Google Scholar] [CrossRef]





| Classification | Representative Solute | Cn | Cu | Cmax | MW (kDa) | Ref |
|---|---|---|---|---|---|---|
| Free water-soluble solute | Urea (g/L) Creatinine (mg/L) | <0.4 <12.0 | 2.3 ± 1.1 136.0 ± 46.0 | 4.6 240.0 | 60 113 | [32,33] |
| Protein-bound solute | Indoxyl sulfate (mg/L) P-cresyl sulfate (mg/L) | 0.6 ± 5.4 0.6 ± 1.0 | 53.0 ± 91.5 20.1 ± 10.5 | 236.0 40.7 | 251 108 | [34,35] |
| Middle molecule | β-2 microglobulin (mg/L) | <2.0 | 55.0 ± 7.9 | 100.0 | 11818 | [36,37] |
| The Pathophysiological Role of Indoxyl Sulfate | Reference |
|---|---|
| Inhibition of endothelial proliferation and wound repair | [2] |
| Progressive deterioration of renal function | [34,46,50,51,52,53,54] |
| Induction of oxidative stress | [55] |
| Increase of circulating EMPs release | [56] |
| Induces TF production via the AhR pathway | [57,58] |
| Development of uremic symptoms | [46,59] |
| Associated with pathogenesis of atherosclerosis | [60] |
| Increases mortality | [61] |
| Cardiovascular disease | [54,61,62,63] |
| Peripheral arterial disease | [61,64,65] |
| The Pathophysiologic Roles of IS | Molecular Mechanisms | References |
|---|---|---|
| Impairment of erythropoiesis in a HIF dependent manner | Suppression of the EPO gene transcription during hypoxia | [43] |
| Stimulates eryptosis | Extracellular Ca2+ entry with subsequent stimulation of cell shrinkage and cell membrane scrambling | [66] |
| Might contribute to EPO resistance and endothelial dysfunction | IS inhibits EPO-Induced Phosphorylation of EPOR IS inhibits TSP-1 expression through suppression of the AKT phosphorylation | [67] |
| Suppression of HIF activation | IS-induced AhR activation | [70] |
| Increased PCA in RBCs | Due to PS exposure and RBCs-derived microparticles release | [71] |
| EPO decrease | IS negatively regulates the EPO expression | [73] |
| IS-induced RBCs death | Through OAT2, and NADPH oxidase activity-dependent, and a GSH-independent mechanism | [75] |
| Current Strategies | Reference |
|---|---|
| ESA | [117,118,119,120,121,122,123,124,125] |
| Iron supplementation | [126,127,128] |
| PHD inhibitors | [91,129,130,131,132] |
| AST-120 | [73,133,134,135,136,137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, E.; Metzinger, L.; Metzinger-Le Meuth, V. Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review. Cells 2020, 9, 2039. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9092039
Hamza E, Metzinger L, Metzinger-Le Meuth V. Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review. Cells. 2020; 9(9):2039. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9092039
Chicago/Turabian StyleHamza, Eya, Laurent Metzinger, and Valérie Metzinger-Le Meuth. 2020. "Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review" Cells 9, no. 9: 2039. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9092039