Addition of Heteroatom Radicals to endo-Glycals †
Abstract
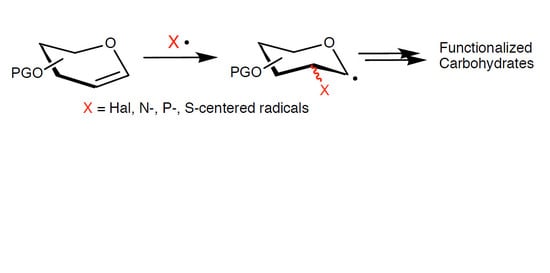
:1. Introduction
2. Addition of Halogen Atoms
3. Addition of Nitrogen-Centered Radicals
4. Addition of Phosphorus-Centered Radicals
5. Addition of Sulfur-Centered Radicals
6. Summary and Perspectives
Funding
Conflicts of Interest
References and Notes
- Fehér, J.; Csomós, G.; Vereckei, A. Free Radical Reactions in Medicine; Springer Science & Business Media: Heidelberg, Germany, 1987. [Google Scholar]
- Nicolaou, K.C.; Dai, W.-M. Chemistry and Biology of the Enediyne Anticancer Antibiotics. Angew. Chem. Int. Ed. 1991, 30, 1387–1416. [Google Scholar] [CrossRef]
- Joshi, M.C.; Rawat, D.S. Recent Developments in Enediyne Chemistry. Chem. Biodivers. 2012, 9, 459–498. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Glofre, S.V.; Chiacchio, M.A. Synthesis and Biological Activity of Unnatural Enediynes. Curr. Med. Chem. 2017, 24, 3433–3484. [Google Scholar] [CrossRef] [PubMed]
- Early book: von Sonntag, C. Radiation Chemistry of Carbohydrates and of the Sugar Moiety in DNA; Elsevier Scientific: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Von Sonntag, C. Weiss Lecture: Carbohydrate Radicals: From Ethylene Glycol to DNA Strand Breakage. Int. J. Radiat. Biol. 1984, 46, 507–519. [Google Scholar] [CrossRef]
- Very recent book: Chadwick, K.H. Understanding Radiation Biology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Giese, B.B.; Be, X.; Burger, J.; Kesselheim, C.; Senn, M.; Schayer, T. The Mechanism of Anaerobic, Radical-Induced DNA Strand Scission. Angew. Chem. Int. Ed. 1993, 2, 1742–1743. [Google Scholar] [CrossRef]
- Giese, B. Hole Injection and Hole Transfer through DNA: The Hopping Mechanism BT - Long-Range Charge Transfer in DNA I; Schuster, G.B., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2004; pp. 27–44. [Google Scholar] [CrossRef]
- First example: Giese, B.; Dupuis, J. Diastereoselective Syntheses of C-Glycopyranosides. Angew. Chem. Int. Ed. 1983, 22, 622–623. [Google Scholar] [CrossRef]
- Giese, B. The Stereoselectivity of Intermolecular Free Radical Reactions. Angew. Chem. Int. Ed. 1989, 8, 969–1146. [Google Scholar] [CrossRef]
- Giese, B.; Dupuis, J.; Gröninger, K.; Haßkerl, T.; Nix, M.; Witzel, T. Orbital Effects in Carbohydrate Radicals. In Substituent Effects in Radical Chemistry; Viehe, H.G., Janousek, Z., Merényi, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1986; pp. 283–296. [Google Scholar] [CrossRef]
- Giese, B. Syntheses with Radicals—C-C Bond Formation via Organotin and Organomercury Compounds. Angew. Chem. Int. Ed. 1985, 24, 553–565. [Google Scholar] [CrossRef]
- Giese, B. Radicals in Organic Synthesis: Formation of Carbon–carbon Bonds; Organic Chemistry Series; Pergamon Press: Oxford, UK, 1986. [Google Scholar]
- Giese, B. Stereoselective Syntheses with Carbohydrate Radicals. Pure Appl. Chem. 1988, 60, 1655–1658. [Google Scholar] [CrossRef]
- Hansen, S.G.; Skrydstrup, T. Modification of Amino Acids, Peptides, and Carbohydrates through Radical Chemistry. In Radicals in Synthesis II; Gansäuer, A., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germnay, 2006; pp. 135–162. [Google Scholar] [CrossRef]
- Pérez-Martín, I.; Suárez, E. Radicals and Carbohydrates. In Encyclopedia of Radicals in Chemistry, Biology and Materials; American Cancer Society: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Very recent book: Binkley, R.W.; Binkley, E.R. Radical Reactions of Carbohydrates. 2019. Available online: http://www.carborad.com (accessed on 20 February 2020).
- Giese, B.; Gröninger, K.S.; Witzel, T.; Korth, H.-G.; Sustmann, R. Synthesis of 2-Deoxy Sugars. Angew. Chem. Int. Ed. 1987, 26, 233–234. [Google Scholar] [CrossRef]
- Giese, B. Formation of CC Bonds by Addition of Free Radicals to Alkenes. Angew. Chemie Int. Ed. 1983, 22, 753–764. [Google Scholar] [CrossRef]
- Praly, J.-P. Structure of Anomeric Glycosyl Radicals and Their Transformations under Reductive Conditions. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: London, UK, 2000; Volume 56, pp. 65–151. [Google Scholar] [CrossRef]
- Very recent review: De Vleeschouwer, F.; Van Speybroeck, V.; Waroquier, M.; Geerlings, P.; De Proft, F. Electrophilicity and Nucleophilicity Index for Radicals. Org. Lett. 2007, 9, 2720–2724. [Google Scholar] [CrossRef]
- Kinfe, H.H. Versatility of Glycals in Synthetic Organic Chemistry: Coupling Reactions, Diversity Oriented Synthesis and Natural Product Synthesis. Org. Biomol. Chem. 2019, 17, 4153–4182. [Google Scholar] [CrossRef] [PubMed]
- First example: Linker, T.; Hartmann, K.; Sommermann, T.; Scheutzow, D.; Ruckdeschel, E. Transition-Metal-Mediated Radical Reactions as an Easy Route to 2-C-Analogues of Carbohydrates. Angew. Chem. Int. Ed. 1996, 35, 1730–1732. [Google Scholar] [CrossRef]
- Linker, T.; Sommermann, T.; Kahlenberg, F. The Addition of Malonates to Glycals: A General and Convenient Method for the Synthesis of 2-C-Branched Carbohydrates. J. Am. Chem. Soc. 1997, 119, 9377–9384. [Google Scholar] [CrossRef]
- Review: Elamparuthi, E.; Kim, B.G.; Yin, J.; Maurer, M.; Linker, T. Cerium(IV)-Mediated C–C Bond Formations in Carbohydrate Chemistry. Tetrahedron 2008, 64, 11925–11937. [Google Scholar] [CrossRef]
- Vankar, Y.D.; Linker, T. Recent Developments in the Synthesis of 2-C-Branched and 1,2-Annulated Carbohydrates. Eur. J. Org. Chem. 2015, 2015, 7633–7642. [Google Scholar] [CrossRef]
- Taniguchi, T. Recent Advances in Reactions of Heteroatom-Centered Radicals. Synthesis 2017, 49, 3511–3534. [Google Scholar] [CrossRef]
- Very recent example: Wu, X.; Zhu, C. Recent Advances in Alkoxy Radical-Promoted C-C and C-H Bond Functionalization Starting from Free Alcohols. Chem. Commun. 2019, 55, 9747–9756. [Google Scholar] [CrossRef]
- De Armas, P.; Francisco, C.G.; Suárez, E. Reagents with Hypervalent Iodine: Formation of Convenient Chiral Synthetic Intermediates by Fragmentation of Carbohydrate Anomeric Alkoxy Radicals. Angew. Chem. Int. Ed. 1992, 31, 772–774. [Google Scholar] [CrossRef]
- Hernández-Guerra, D.; Rodríguez, M.S.; Suárez, E. Fragmentation of Carbohydrate Anomeric Alkoxyl Radicals: Synthesis of Chiral Polyhydroxylated β-Iodo- and Alkenylorganophosphorus(V) Compounds. Eur. J. Org. Chem. 2014, 2014, 5033–5055. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Fraser-Reid, B. The Mechanisms of the Halogenations and Halogenomethoxylations of D-Glucal Triacetate, D-Galactal Triacetate, and 3,4-Dihydropyran. Can. J. Chem. 1965, 43, 1460–1475. [Google Scholar] [CrossRef]
- Hassner, A. Regiospecific and Stereospecific Introduction of Azide Functions into Organic Molecules. Acc. Chem. Res. 1971, 4, 9–16. [Google Scholar] [CrossRef]
- Bovin, N.V.; Zurabyan, S.É.; Khorlin, A.Y. Addition of Halogenoazides to Glycals. Carbohydr. Res. 1981, 98, 25–35. [Google Scholar] [CrossRef]
- Rawal, G.K.; Kumar, A.; Tawar, U.; Vankar, Y.D. New Method for Chloroamidation of Olefins. Application in the Synthesis of N-Glycopeptides and Anticancer Agents. Org. Lett. 2007, 9, 5171–5174. [Google Scholar] [CrossRef]
- Kirschning, A.; Jesberger, M.; Schönberger, A. The First Polymer-Assisted Solution-Phase Synthesis of Deoxyglycosides. Org. Lett. 2001, 3, 3623–3626. [Google Scholar] [CrossRef]
- Islam, M.; Tirukoti, N.D.; Nandi, S.; Hotha, S. Hypervalent Iodine Mediated Synthesis of C-2 Deoxy Glycosides and Amino Acid Glycoconjugates. J. Org. Chem. 2014, 79, 4470–4476. [Google Scholar] [CrossRef]
- Kundoor, G.; Rao, D.S.; Kashyap, S. Regioselective Direct Difunctionalization of Glycals: Convenient Access to 2-Deoxyglycoconjugates Mediated by Tetra-n-Butylammonium Iodide/Sodium Periodate. Asian J. Org. Chem. 2016, 5, 264–270. [Google Scholar] [CrossRef]
- Nair, V.; Deepthi, A. Cerium(IV) Ammonium Nitrate—A Versatile Single-Electron Oxidant. Chem. Rev. 2007, 107, 1862–1891. [Google Scholar] [CrossRef]
- Linker, T.; Schanzenbach, D.; Elamparuthi, E.; Sommermann, T.; Fudickar, W.; Gyóllai, V.; Somsák, L.; Demuth, W.; Schmittel, M. Remarkable Oxidation Stability of Glycals: Excellent Substrates for Cerium(IV)-Mediated Radical Reactions. J. Am. Chem. Soc. 2008, 130, 16003–16010. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, R.M.; Lemieux, R.U. The Azidonitration of Tri-O-Acetyl-D-Galactal. Can. J. Chem. 1979, 57, 1244–1251. [Google Scholar]
- Paulsen, H.; Lorentzen, J.P.; Kutschker, W. Erprobte Synthese von 2-Azido-2-Desoxy-D-Mannose Und 2-Azido-2-Desoxy-D-Mannuronsäure Als Baustein Zum Aufbau von Bakterien-Polysaccharid-Sequenzen. Carbohydr. Res. 1985, 136, 153–176. [Google Scholar] [CrossRef]
- Kinzy, W.; Schmidt, R.R. Glycosylimidate, 16. Synthese Des Trisaccharids Aus Der “Repeating Unit” Des Kapselpolysaccharids von Neisseria Meningitidis (Serogruppe L). Liebigs Ann. Chem. 1985, 1985, 1537–1545. [Google Scholar] [CrossRef]
- Keith, D.J.; Townsend, S.D. Total Synthesis of the Congested, Bisphosphorylated Morganella Morganii Zwitterionic Trisaccharide Repeating Unit. J. Am. Chem. Soc. 2019, 141, 12939–12945. [Google Scholar] [CrossRef]
- Singh, Y.; Wang, T.; Demchenko, A.V. Direct Glycosidation of 2-Azido-2-Deoxyglycosyl Nitrates. Eur. J. Org. Chem. 2019, 2019, 6413–6416. [Google Scholar] [CrossRef]
- Czernecki, S.; Randriamandimby, D. Azido-Phenylselenylation of Protected Glycals. Tetrahedron Lett. 1993, 34, 7915–7916. [Google Scholar] [CrossRef]
- Santoyo-González, F.; Calvo-Flores, F.G.; García-Mendoza, P.; Hernández-Mateo, F.; Isac-García, J.; Robles-Díaz, R. Synthesis of Phenyl 2-Azido-2-Deoxy-1-Selenoglycosides from Glycals. J. Org. Chem. 1993, 58, 6122–6125. [Google Scholar] [CrossRef]
- Jiaang, W.T.; Chang, M.Y.; Tseng, P.H.; Chen, S.T. A Concise Synthesis of the O-Glycosylated Amino Acid Building Block; Using Phenyl Selenoglycoside as a Glycosyl Donor. Tetrahedron Lett. 2000, 41, 3127–3130. [Google Scholar] [CrossRef]
- Churchill, D.G.; Rojas, C.M. Iron(II)-Promoted Amidoglycosylation and Amidochlorination of an Allal C3-Azidoformate. Tetrahedron Lett. 2002, 43, 7225–7228. [Google Scholar] [CrossRef]
- Lu, D.F.; Zhu, C.L.; Jia, Z.X.; Xu, H. Iron(II)-Catalyzed Intermolecular Amino-Oxygenation of Olefins through the N-O Bond Cleavage of Functionalized Hydroxylamines. J. Am. Chem. Soc. 2014, 136, 13186–13189. [Google Scholar] [CrossRef] [Green Version]
- Leca, D.; Fensterbank, L.; Lacôte, E.; Malacria, M. Recent Advances in the Use of Phosphorus-Centered Radicals in Organic Chemistry. Chem. Soc. Rev. 2005, 34, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent Advances in Sulfur- and Phosphorous-Centered Radical Reactions for the Formation of S–C and P–C Bonds. Tetrahedron 2015, 71, 7481–7529. [Google Scholar] [CrossRef]
- Kazuo, K.; Yoshida, H.; Ogata, T.; Inokawa, S. Sugars Containing a Carbon-Phosphorus Bond. I. Photochemical Addition of Diethyl Thiophosphonate to Unsaturated Sugars. Bull. Chem. Soc. Jpn. 1969, 42, 3245–3248. [Google Scholar]
- Jessop, C.M.; Parsons, A.F.; Routledge, A.; Irvine, D.J. Phosphonyl Radical Addition to Enol Ethers. The Stereoselective Synthesis of Cyclic Ethers. Tetrahedron Lett. 2004, 45, 5095–5098. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Zhang, C.; Zhao, Y. Novel Regio- and Stereoselective Phosphonyl Radical Addition to Glycals Promoted by Mn(II)-Air: Syntheses of 1,2-Dideoxy 2-C- Diphenylphosphinylglycopyranosides. Chem. Commun. 2014, 50, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Elamparuthi, E.; Linker, T. Carbohydrate-2-Deoxy-2-Phosphonates: Simple Synthesis and Horner-Emmons Reaction. Angew. Chem. Int. Ed. 2009, 48, 1853–1855. [Google Scholar] [CrossRef]
- Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol-Enes: Chemistry of the Past with Promise for the Future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Fiore, M.; Marra, A.; Dondoni, A. Photoinduced Thiol-Ene Coupling as a Click Ligation Tool for Thiodisaccharide Synthesis. J. Org. Chem. 2009, 74, 4422–4425. [Google Scholar] [CrossRef]
- McSweeney, L.; Dénès, F.; Scanlan, E.M. Thiyl-Radical Reactions in Carbohydrate Chemistry: From Thiosugars to Glycoconjugate Synthesis. Eur. J. Org. Chem. 2016, 2016, 2080–2095. [Google Scholar] [CrossRef]
- Igarashi, K.; Honma, T. Addition Reactions of Glycals. IV. Free-Radical Addition of Thiolacetic Acid to D-Glucal Triacetate. J. Org. Chem. 1970, 35, 606–610. [Google Scholar] [CrossRef]
- Araki, Y.; Matsuura, K.; Ishido, Y.; Kushida, K. Synthetic Studies of Carbohydrate Derivatives with Photochemical Reaction. VII. Photochemical Addition of Ethanethiol and 1-Propanethiol to Enoses. Chem. Lett. 1973, 2, 383–386. [Google Scholar] [CrossRef]
- Staderini, S.; Chambery, A.; Marra, A.; Dondoni, A. Free-Radical Hydrothiolation of Glycals: A Thiol-Ene-Based Synthesis of S-Disaccharides. Tetrahedron Lett. 2012, 53, 702–704. [Google Scholar] [CrossRef]
- Lázár, L.; Csávás, M.; Herczeg, M.; Herczegh, P.; Borbás, A. Synthesis of S-Linked Glycoconjugates and S-Disaccharides by Thiol–Ene Coupling Reaction of Enoses. Org. Lett. 2012, 14, 4650–4653. [Google Scholar] [CrossRef]
- Lázár, L.; Csávás, M.; Hadházi, Á.; Herczeg, M.; Tóth, M.; Somsák, L.; Barna, T.; Herczegh, P.; Borbás, A. Systematic Study on Free Radical Hydrothiolation of Unsaturated Monosaccharide Derivatives with Exo- and Endocyclic Double Bonds. Org. Biomol. Chem. 2013, 11, 5339–5350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavashe, P.; Elamparuthi, E.; Hettrich, C.; Möller, H.M.; Linker, T. Synthesis of 2-Thiocarbohydrates and Their Binding to Concanavalin A. J. Org. Chem. 2016, 81, 8595–8603. [Google Scholar] [CrossRef]
- Fudickar, W.; Pavashe, P.; Linker, T. Thiocarbohydrates on Gold Nanoparticles: Strong Influence of Stereocenters on Binding Affinity and Interparticle Forces. Chem. Eur. J. 2017, 23, 8685–8693. [Google Scholar] [CrossRef]
- Lázár, L.; Borbás, A.; Somsák, L. Synthesis of Thiomaltooligosaccharides by a Thio-Click Approach. Carbohydr. Res. 2018, 470, 8–12. [Google Scholar] [CrossRef]
- Eszenyi, D.; Kelemen, V.; Balogh, F.; Bege, M.; Csávás, M.; Herczegh, P.; Borbás, A. Promotion of a Reaction by Cooling: Stereoselective 1,2-Cis-α-Thioglycoconjugation by Thiol-Ene Coupling at −80 °C. Chem. Eur. J. 2018, 24, 4532–4536. [Google Scholar] [CrossRef]
- Review: Dondoni, A.; Marra, A. Recent Applications of Thiol-Ene Coupling as a Click Process for Glycoconjugation. Chem. Soc. Rev. 2012, 41, 573–586. [Google Scholar] [CrossRef]
- Lázár, L.; Juhász, L.; Batta, G.; Borbás, A.; Somsák, L. Unprecedented β-Manno Type Thiodisaccharides with a C-Glycosylic Function by Photoinitiated Hydrothiolation of 1-C-Substituted Glycals. New J. Chem. 2017, 41, 1284–1292. [Google Scholar] [CrossRef] [Green Version]
- Very recent example: Kelemen, V.; Bege, M.; Eszenyi, D.; Debreczeni, N.; Bényei, A.; Stürzer, T.; Herczegh, P.; Borbás, A. Stereoselective Thioconjugation by Photoinduced Thiol-Ene Coupling Reactions of Hexo- and Pentopyranosyl D- and L-Glycals at Low-Temperature—Reactivity and Stereoselectivity Study. Chem. Eur. J. 2019, 25, 14555–14571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollivier, C.; Renaud, P. Organoboranes as a Source of Radicals. Chem. Rev. 2001, 101, 3415–3434. [Google Scholar] [CrossRef] [PubMed]
- Renaud, P. Boron in Radical Chemistry. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Taniguchi, T. Boryl Radical Addition to Multiple Bonds in Organic Synthesis. Eur. J. Org. Chem. 2019, 2019, 6308–6319. [Google Scholar] [CrossRef]


















© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linker, T.
Addition of Heteroatom Radicals to endo-Glycals
Linker T.
Addition of Heteroatom Radicals to endo-Glycals
Linker, Torsten.
2020. "Addition of Heteroatom Radicals to endo-Glycals