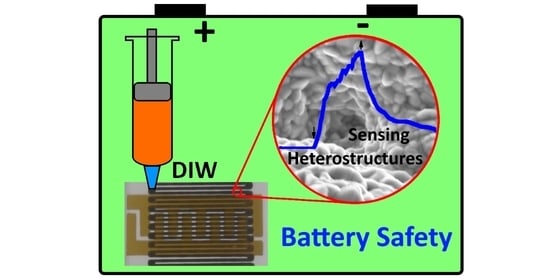
Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. 3D-DIW Chemosensors Based on Semiconducting Oxide Nanostructures on CuO/Cu2O/Cu Heterojunction Net with Al2O3 Coating for Battery Safety Applications
3.2. Characteristics of Semiconducting Oxide Nanostructures by Direct Ink Writing of Metal Microparticles and Their Potential Application as Battery Safety Sensors
4. New Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharafeldin, M.; Kadimisetty, K.; Bhalerao, K.S.; Chen, T.; Rusling, J.F. 3D-Printed Immunosensor Arrays for Cancer Diagnostics. Sensors 2020, 20, 4514. [Google Scholar] [CrossRef]
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro additive manufacturing of glucose biosensors: A feasibility study. Anal. Chim. Acta 2018, 1043, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liyarita, B.R.; Ambrosi, A.; Pumera, M. 3D-printed Electrodes for Sensing of Biologically Active Molecules. Electroanalysis 2018, 30, 1319–1326. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. Single-step fabrication of an integrated 3D-printed device for electrochemical sensing applications. Electrochem. Commun. 2019, 103, 100–103. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; de Aparecido, G.O.; de Ferraz, T.V.B.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.H.; Chua, C.K.; Pumera, M. DNA biosensing with 3D printing technology. Analyst 2017, 142, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Kadimisetty, K.; Bhalerao, K.R.; Bist, I.; Jones, A.; Chen, T.; Lee, N.H.; Rusling, J.F. Accessible Telemedicine Diagnostics with ELISA in a 3D Printed Pipette Tip. Anal. Chem. 2019, 91, 7394–7402. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, 2004782. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Mukhopadhyay, S.C. Occupancy Detection at Smart Home Using Real-Time Dynamic Thresholding of Flexiforce Sensor. IEEE Sens. J. 2015, 15, 4457–4463. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Lu, D.; Lin, S.; Hu, S.; Cui, W.; Fang, T.; Iqbal, A.; Zhang, Z.; Peng, W. Thermal behavior and failure mechanism of large format lithium-ion battery. J. Solid State Electrochem. 2021, 25, 315–325. [Google Scholar] [CrossRef]
- Essl, C.; Seifert, L.; Rabe, M.; Fuchs, A. Early Detection of Failing Automotive Batteries Using Gas Sensors. Batteries 2021, 7, 25. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Lin, K.H.; Kao, C.-S. Experimental investigation and visualization on thermal runaway of hard prismatic lithium-ion batteries used in smart phones. J. Therm. Anal. Calorim. 2018, 132, 1677–1692. [Google Scholar] [CrossRef]
- Vahl, A.; Lupan, O.; Santos-Carballal, D.; Postica, V.; Hansen, S.; Cavers, H.; Wolff, N.; Terasa, M.-I.; Hoppe, M.; Cadi-Essadek, A.; et al. Surface functionalization of ZnO:Ag columnar thin films with AgAu and AgPt bimetallic alloy nanoparticles as an efficient pathway for highly sensitive gas discrimination and early hazard detection in batteries. J. Mater. Chem. A 2020, 8, 16246–16264. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of Thermal Runaway in Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A1303–A1308. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Chen, Z.; Hsu, P.-C.; Lopez, J.; Li, Y.; To, J.W.F.; Liu, N.; Wang, C.; Andrews, S.C.; Liu, J.; Cui, Y.; et al. Fast and reversible thermoresponsive polymer switching materials for safer batteries. Nat. Energy 2016, 1, 15009. [Google Scholar] [CrossRef]
- Siebert, L.; Wolff, N.; Ababii, N.; Terasa, M.-I.; Lupan, O.; Vahl, A.; Duppel, V.; Qiu, H.; Tienken, M.; Mirabelli, M.; et al. Facile fabrication of semiconducting oxide nanostructures by direct ink writing of readily available metal microparticles and their application as low power acetone gas sensors. Nano Energy 2020, 70, 104420. [Google Scholar] [CrossRef]
- Cai, T.; Valecha, P.; Tran, V.; Engle, B.; Stefanopoulou, A.; Siegel, J. Detection of Li-ion battery failure and venting with Carbon Dioxide sensors. eTransportation 2021, 7, 100100. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D Printing of Strain Sensors within Highly Stretchable Elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Yang, C.; Hsu, W.; Lin, L. 3D-printed microelectronics for integrated circuitry and passive wireless sensors. Microsyst. Nanoeng. 2015, 1, 15013. [Google Scholar] [CrossRef] [Green Version]
- Siebert, L.; Lupan, O.; Mirabelli, M.; Ababii, N.; Terasa, M.-I.; Kaps, S.; Cretu, V.; Vahl, A.; Faupel, F.; Adelung, R. 3D-Printed Chemiresistive Sensor Array on Nanowire CuO/Cu2O/Cu Heterojunction Nets. ACS Appl. Mater. Interfaces 2019, 11, 25508–25515. [Google Scholar] [CrossRef]
- Naik, K.M.; Higuchi, E.; Inoue, H. Two-dimensional oxygen-deficient TiO2 nanosheets-supported Pt nanoparticles as durable catalyst for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2020, 455, 227972. [Google Scholar] [CrossRef]
- Kukunuri, S.; Naik, K.; Sampath, S. Effects of composition and nanostructuring of palladium selenide phases, Pd4Se, Pd7Se4 and Pd17Se15, on ORR activity and their use in Mg–air batteries. J. Mater. Chem. A 2017, 5, 4660–4670. [Google Scholar] [CrossRef]
- Lupan, O.; Santos-Carballal, D.; Ababii, N.; Magariu, N.; Hansen, S.; Vahl, A.; Zimoch, L.; Hoppe, M.; Pauporté, T.; Galstyan, V.; et al. TiO2/Cu2O/CuO Multi-Nanolayers as Sensors for H2 and Volatile Organic Compounds: An Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2021, 13, 32363–32380. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science. 2016, 353, aaf2093. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Boydston, A.J. Multimaterial actinic spatial control 3D and 4D printing. Nat. Commun. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Nadgorny, M.; Ameli, A. Functional Polymers and Nanocomposites for 3D Printing of Smart Structures and Devices. ACS Appl. Mater. Interfaces 2018, 10, 17489–17507. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Guo, X.; Kong, B.; Zhang, M.; Qian, X.; Mi, S.; Sun, W. The Boom in 3D-Printed Sensor Technology. Sensors 2017, 17, 1166. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., Ed.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 128. [Google Scholar]
- Vahl, A.; Dittmann, J.; Jetter, J.; Veziroglu, S.; Shree, S.; Ababii, N.; Lupan, O.; Aktas, O.C.; Strunskus, T.; Quandt, E.; et al. The impact of O2/Ar ratio on morphology and functional properties in reactive sputtering of metal oxide thin films. Nanotechnology 2019, 30, 235603. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Bodduluri, M.T.; Magariu, N.; Vahl, A.; Krüger, H.; Wagner, B.; Faupel, F.; Adelung, R.; et al. Heterostructure-based devices with enhanced humidity stability for H2 gas sensing applications in breath tests and portable batteries. Sens. Actuators A Phys. 2021, 329, 112804. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Gronenberg, O.; Vahl, A.; Schürmann, U.; Duppel, V.; Krüger, H.; Chow, L.; Kienle, L.; et al. Single CuO/Cu2O/Cu Microwire Covered by a Nanowire Network as a Gas Sensor for the Detection of Battery Hazards. ACS Appl. Mater. Interfaces 2020, 12, 42248–42263. [Google Scholar] [CrossRef]
- Lupan, O.; Cretu, V.; Postica, V.; Polonskyi, O.; Ababii, N.; Schütt, F.; Kaidas, V.; Faupel, F.; Adelung, R. Non-planar nanoscale p–p heterojunctions formation in ZnxCu1-xOy nanocrystals by mixed phases for enhanced sensors. Sens. Actuators B Chem. 2016, 230, 832–843. [Google Scholar] [CrossRef]
- Lupan, O.; Cretu, V.; Postica, V.; Ababii, N.; Polonskyi, O.; Kaidas, V.; Schütt, F.; Mishra, Y.K.; Monaico, E.; Tiginyanu, I.; et al. Enhanced ethanol vapour sensing performances of copper oxide nanocrystals with mixed phases. Sens. Actuators B Chem. 2016, 224, 434–448. [Google Scholar] [CrossRef]
- Cretu, V.; Postica, V.; Mishra, A.K.; Hoppe, M.; Tiginyanu, I.; Mishra, Y.K.; Chow, L.; de Leeuw, N.H.; Adelung, R.; Lupan, O. Synthesis, characterization and DFT studies of zinc-doped copper oxide nanocrystals for gas sensing applications. J. Mater. Chem. A 2016, 4, 6527–6539. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Bedi, R.K. Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl. Surf. Sci. 2011, 257, 7592–7599. [Google Scholar] [CrossRef]
- Lupan, C.; Khaledialidusti, R.; Mishra, A.K.; Postica, V.; Terasa, M.-I.; Magariu, N.; Pauporté, T.; Viana, B.; Drewes, J.; Vahl, A.; et al. Pd-Functionalized ZnO:Eu Columnar Films for Room-Temperature Hydrogen Gas Sensing: A Combined Experimental and Computational Approach. ACS Appl. Mater. Interfaces 2020, 12, 24951–24964. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Irwin, J.C. Raman scattering from cupric oxide. Solid State Commun. 1989, 70, 11–14. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 26 March 2020).
- Lupan, O.; Postica, V.; Ababii, N.; Hoppe, M.; Cretu, V.; Tiginyanu, I.; Sontea, V.; Pauporté, T.; Viana, B.; Adelung, R. Influence of CuO nanostructures morphology on hydrogen gas sensing performances. Microelectron. Eng. 2016, 164, 63–70. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Petrunin, V.F. Development of Nanomaterials for Nuclear Energetics. Phys. Procedia 2015, 72, 536–539. [Google Scholar] [CrossRef] [Green Version]
- Baloyan, B.M.; Kolmakov, А.G.; Alymov, М.I.; Krotov, А.М. Nanomaterials Classification, Property Features, Application and Production Technology; International University of Nature, Society and Man Dubna: Ugresha, Russia, 2007; p. 125. [Google Scholar]
- Intaniwet, A.; Mills, C.A.; Shkunov, M.; Sellin, P.J.; Keddie, J.L. Heavy metallic oxide nanoparticles for enhanced sensitivity in semiconducting polymer x-ray detectors. Nanotechnology 2012, 23, 235502. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, E.A.; Harbi, H.F.A.L.; Aref, N. Radioprotective efficacy of zinc oxide nanoparticles on γ-ray-induced nuclear DNA damage in Vicia faba L. as evaluated by DNA bioassays. J. Radiat. Res. Appl. Sci. 2019, 12, 423–436. [Google Scholar] [CrossRef]
- Sahani, R.M.; Kumari, C.; Pandya, A.; Dixit, A. Efficient Alpha Radiation Detector using Low Temperature Hydrothermally Grown ZnO:Ga Nanorod Scintillator. Sci. Rep. 2019, 9, 11354. [Google Scholar] [CrossRef]
- Valença, C.P.V.; Silveira, M.A.L.; Macedo, M.A.; Santos, M.A. Energy dependence evaluation of a ZnO detector for diagnostic X-ray beam. In Proceedings of the 10 Latin American Regional Congress IRPA Protection and Radiation Safety, Buenos Aires, Argentina, 12–17 April 2015; p. 14. [Google Scholar]
- Abe, T.; Suzuki, Y.; Nakagawa, A.; Chiba, T.; Nakagawa, M.; Kashiwaba, Y.; Niikura, I.; Kashiwaba, Y.; Osada, H. Application of a ZnO UV sensor for a scintillation-type radiation detector. J. Mater. Sci. Mater. Electron. 2019, 30, 16873–16877. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Baffa, O.; Clarke, D.R. Enhanced UV Emission from Silver/ZnO And Gold/ZnO Core-Shell Nanoparticles: Photoluminescence, Radioluminescence, And Optically Stimulated Luminescence. Sci. Rep. 2015, 5, 14004. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, A.; Chatterton, N.; Crabb, E.M.; Golding, J.P. A comparison of the radiosensitisation ability of 22 different element metal oxide nanoparticles using clinical megavoltage X-rays. Cancer Nanotechnol. 2019, 10, 10. [Google Scholar] [CrossRef]












| Sample Set | 3D-T | 3D-L |
|---|---|---|
| Base solution PEO content | 6% | 6.5% |
| Trace height | >0.2 mm | 0.2 mm |
| Trace width | >0.6 mm | 0.6 mm |
| Trace orientation | transversal | longitudinal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupan, O.; Krüger, H.; Siebert, L.; Ababii, N.; Kohlmann, N.; Buzdugan, A.; Bodduluri, M.T.; Magariu, N.; Terasa, M.-I.; Strunskus, T.; et al. Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring. Chemosensors 2021, 9, 252. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9090252
Lupan O, Krüger H, Siebert L, Ababii N, Kohlmann N, Buzdugan A, Bodduluri MT, Magariu N, Terasa M-I, Strunskus T, et al. Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring. Chemosensors. 2021; 9(9):252. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9090252
Chicago/Turabian StyleLupan, Oleg, Helge Krüger, Leonard Siebert, Nicolai Ababii, Niklas Kohlmann, Artur Buzdugan, Mani Teja Bodduluri, Nicolae Magariu, Maik-Ivo Terasa, Thomas Strunskus, and et al. 2021. "Additive Manufacturing as a Means of Gas Sensor Development for Battery Health Monitoring" Chemosensors 9, no. 9: 252. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9090252