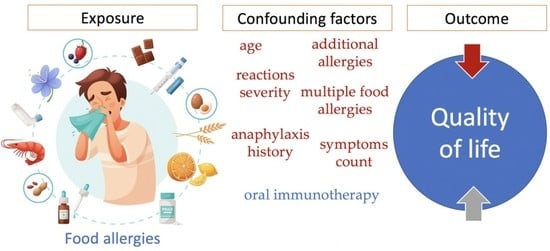
Food Allergies and Quality of Life among School-Aged Children and Adolescents: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Eligibility Criteria
2.3. Quality Assessment
2.4. Data Collection Process
2.5. Compliance with Ethics Guidelines
3. Results
3.1. Eligible Studies
3.2. Characteristics of Eligible Studies and Population
3.3. Quality of Life of Children and Adolescents with Food Allergy in Observational Studies
3.4. Quality of Life of Children and Adolescents with Food Allergy in Interventional Studies
3.5. Confounding Factors That Affect HRQoL of Children and Adolescents with Food Allergy
3.6. Quality Assessment of the Reviewed Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silverstein, A.M. Clemens Freiherr von Pirquet: Explaining Immune Complex Disease in 1906. Nat. Immunol. 2000, 1, 453–455. [Google Scholar] [CrossRef]
- Boardman, A.; Knight, K.; Kane, P.; Fitzsimons, R. Recognition and Management of Food Allergy in Children. Nurs. Child. Young People 2019, 31, 21–26. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International Consensus on (ICON) Anaphylaxis. World Allergy Organ. J. 2014, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Ruiz Sánchez, J.G.; Palma Milla, S.; Pelegrina Cortés, B.; López Plaza, B.; Bermejo López, L.M.; Gómez-Candela, C. A global vision of adverse reactions to foods: Food allergy and food intolerance. Nutr. Hosp. 2018, 35, 102–108. [Google Scholar] [CrossRef]
- Szépfalusi, Z.; Spiesz, K.; Huttegger, I. Diagnostik Und Management von Nahrungsmittelallergien Im Kindes-Und Jugendalter. Wien. Med. Wochenschr. 2015, 165, 354–360. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T.; Ebisawa, M.; Ito, K.; Fujisawa, T.; Aihara, Y.; Ito, S.; Imai, T.; Ohshima, Y.; et al. Japanese Guidelines for Food Allergy 2020. Allergol. Int. 2020, 69, 370–386. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Makarova, S.G.; Namazova-Baranova, L.S.; Vishneva, E.A.; Gevorkyan, A.K.; Alekseeva, A.A.; Petrovskaya, M.I. Topical Issues of Food Allergy Diagnosis in Pediatric Practice. Ann. Russ. Acad. Med. Sci. 2015, 70, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Foong, R.-X.; Santos, A.F. Biomarkers of Diagnosis and Resolution of Food Allergy. Pediatr. Allergy Immunol. 2020, 32, 223–233. [Google Scholar] [CrossRef]
- Cox, A.L.; Nowak-Wegrzyn, A. Innovation in Food Challenge Tests for Food Allergy. Curr. Allergy Asthma Rep. 2018, 18, 74. [Google Scholar] [CrossRef]
- Greiwe, J. Oral Food Challenges in Infants and Toddlers. Immunol. Allergy Clin. N. Am. 2019, 39, 481–493. [Google Scholar] [CrossRef]
- Lieberman, J.; Sublett, J.; Ali, Y.; Haselkorn, T.; Damle, V.; Chidambaram, A.; Rosen, K.; Mahr, T. Increased incidence and prevalence of peanut allergy in children and adolescents in the united states. Ann. Allergy Asthma Immunol. 2018, 121, S13. [Google Scholar] [CrossRef]
- McGowan, E.C.; Keet, C.A. Prevalence of Self-Reported Food Allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J. Allergy Clin. Immunol. 2013, 132, 1216–1219.e5. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.-L.; Tang, M.L.K.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. The Prevalence of Food Allergy and Other Allergic Diseases in Early Childhood in a Population-Based Study: HealthNuts Age 4-Year Follow-Up. J. Allergy Clin. Immunol. 2017, 140, 145–153.e8. [Google Scholar] [CrossRef] [Green Version]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and Natural History of Challenge-Proven Cow’s Milk Allergy in European Children—EuroPrevall Birth Cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Motosue, M.S.; Bellolio, M.F.; Houten, H.K.V.; Shah, N.D.; Campbell, R.L. National Trends in Emergency Department Visits and Hospitalizations for Food-Induced Anaphylaxis in US Children. Pediatr. Allergy Immunol. 2018, 29, 538–544. [Google Scholar] [CrossRef]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Davis, C.M.; Kelso, J.M. Food Allergy Management. Immunol. Allergy Clin. N. Am. 2018, 38, 53–64. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: Epidemiology, Pathogenesis, Diagnosis, and Treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef]
- Mehr, S.; Robinson, M.; Tang, M. Doctor? How Do I Use My EpiPen? Pediatr. Allergy Immunol. 2007, 18, 448–452. [Google Scholar] [CrossRef]
- Jones, C.J.; Llewellyn, C.D.; Frew, A.J.; Toit, G.D.; Mukhopadhyay, S.; Smith, H. Factors Associated with Good Adherence to Self-Care Behaviours amongst Adolescents with Food Allergy. Pediatr. Allergy Immunol. 2015, 26, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Licari, A.; Manti, S.; Marseglia, A.; Brambilla, I.; Votto, M.; Castagnoli, R.; Leonardi, S.; Marseglia, G.L. Food Allergies: Current and Future Treatments. Medicina 2019, 55, 120. [Google Scholar] [CrossRef] [Green Version]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen Immunotherapy for IgE-Mediated Food Allergy: A Systematic Review and Meta-Analysis. Allergy 2017, 72, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.M.; Burks, A.W.; Dupont, C. State of the Art on Food Allergen Immunotherapy: Oral, Sublingual, and Epicutaneous. J. Allergy Clin. Immunol. 2014, 133, 318–323. [Google Scholar] [CrossRef]
- Yee, C.S.K.; Rachid, R. The Heterogeneity of Oral Immunotherapy Clinical Trials: Implications and Future Directions. Curr. Allergy Asthma Rep. 2016, 16, 25. [Google Scholar] [CrossRef]
- Albuhairi, S.; Rachid, R. Novel Therapies for Treatment of Food Allergy. Immunol. Allergy Clin. N. Am. 2020, 40, 175–186. [Google Scholar] [CrossRef]
- Siegrist, J.; Junge, A. Conceptual and Methodological Problems in Research on the Quality of Life in Clinical Medicine. Soc. Sci. Med. 1989, 29, 463–468. [Google Scholar] [CrossRef]
- World Health Organization, Division of Mental Health and Prevention of Substance Abuse. WHOQOL: Measuring Quality of Life; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Galvin, A.D.; Hourihane, J.O. Health-Related Quality of Life in Food Allergy. Bundesgesundheitsblatt—Gesundh.—Gesundh. 2016, 59, 841–848. [Google Scholar] [CrossRef]
- Polk, B.I.; Dinakar, C. Patient-Centered Outcomes in Food Allergy. Curr. Allergy Asthma Rep. 2017, 17, 39. [Google Scholar] [CrossRef]
- Feng, C.; Kim, J.-H. Beyond Avoidance: The Psychosocial Impact of Food Allergies. Clin. Rev. Allergy Immunol. 2018, 57, 74–82. [Google Scholar] [CrossRef]
- Springston, E.E.; Smith, B.; Shulruff, J.; Pongracic, J.; Holl, J.; Gupta, R.S. Variations in Quality of Life among Caregivers of Food Allergic Children. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2010, 105, 287–294.e3. [Google Scholar] [CrossRef]
- Patel, N.; Herbert, L.; Green, T.D. The Emotional, Social, and Financial Burden of Food Allergies on Children and Their Families. Allergy Asthma Proc. 2017, 38, 88–91. [Google Scholar] [CrossRef]
- Sharma, H.P.; Herbert, L.J. Food Allergy: Psychosocial Impact and Public Policy Implications. In Food Allergy: Molecular Basis and Clinical Practice; Ebisawa, M., Ballmer-Weber, B.K., Vieths, S., Wood, R.A., Eds.; S. Karger AG: Basel, Switzerland, 2015; Volume 101, pp. 221–226. [Google Scholar]
- Bollinger, M.E.; Dahlquist, L.M.; Mudd, K.; Sonntag, C.; Dillinger, L.; McKenna, K. The Impact of Food Allergy on the Daily Activities of Children and Their Families. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2006, 96, 415–421. [Google Scholar] [CrossRef]
- Walkner, M.; Warren, C.; Gupta, R.S. Quality of Life in Food Allergy Patients and Their Families. Pediatr. Clin. N. Am. 2015, 62, 1453–1461. [Google Scholar] [CrossRef]
- Stensgaard, A.; Bindslev-Jensen, C.; Nielsen, D.; Munch, M.; DunnGalvin, A. Quality of Life in Childhood, Adolescence and Adult Food Allergy: Patient and Parent Perspectives. Clin. Exp. Allergy 2016, 47, 530–539. [Google Scholar] [CrossRef]
- Middelveld, R.; Gunnarsson, N.V.; Ahlstedt, S.; Protudjer, J.L.P. Associations between Food Allergy and Perceived Life Status. Ann. Allergy Asthma Immunol. 2020, 125, 703–705.e1. [Google Scholar] [CrossRef]
- Thörnqvist, V.; Middelveld, R.; Wai, H.M.; Ballardini, N.; Nilsson, E.; Strömquist, J.; Ahlstedt, S.; Nilsson, L.J.; Protudjer, J.L.P. Health-Related Quality of Life Worsens by School Age amongst Children with Food Allergy. Clin. Transl. Allergy 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Morou, Z.; Tatsioni, A.; Dimoliatis, I.D.K.; Papadopoulos, N.G. Health-Related Quality of Life in Children with Food Allergy and Their Parents: A Systematic Review of the Literature. J. Investig. Allergol. Clin. Immunol. 2014, 24, 382–395. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Protudjer, J.L.P.; Middelveld, R.; Dahlén, S.E.; Ahlstedt, S.; FoodHE Investigators. Food Allergy-Related Concerns during the Transition to Self-Management. Allergy Asthma Clin. Immunol. 2019, 15, 54. [Google Scholar] [CrossRef]
- Protudjer, J.L.P.; Jansson, S.-A.; Middelveld, R.; Östblom, E.; Dahlén, S.-E.; Arnlind, M.H.; Bengtsson, U.; Kallström-Bengtsson, I.; Marklund, B.; Rentzos, G.; et al. Impaired Health-Related Quality of Life in Adolescents with Allergy to Staple Foods. Clin. Transl. Allergy 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Strinnholm, Å.; Hedman, L.; Winberg, A.; Jansson, S.-A.; Lindh, V.; Rönmark, E. Health Related Quality of Life among Schoolchildren Aged 12–13 Years in Relation to Food Hypersensitivity Phenotypes: A Population-Based Study. Clin. Transl. Allergy 2017, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Protudjer, J.L.P.; Jansson, S.-A.; Östblom, E.; Arnlind, M.H.; Bengtsson, U.; Dahlén, S.-E.; Kallström-Bengtsson, I.; Marklund, B.; Middelveld, R.J.M.; Rentzos, G.; et al. Health-Related Quality of Life in Children with Objectively Diagnosed Staple Food Allergy Assessed with a Disease-Specific Questionnaire. Acta Paediatr. 2015, 104, 1047–1054. [Google Scholar] [CrossRef]
- Manso, L.; Pineda, R.; Huertas, B.; Fernández-Rivas, M.; Diéguez, M.C.; Cerecedo, I.; Muriel, A.; Fernández, F.B.; DunnGalvin, A.; Antolín-Amérigo, D.; et al. Validation of the Spanish Version of the Food Allergy Quality of Life Questionnaire-Parent Form (S-FAQLQ-PF). J. Investig. Allergol. Clin. Immunol. 2017, 27, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Ortiz, M.; Alvaro, M.; Piquer, M.; Dominguez, O.; Giner, M.T.; Lozano, J.; Jiménez-Feijoo, R.; Plaza, A.M. Impact of Oral Immunotherapy on Quality of Life in Egg-Allergic Children. Pediatr. Allergy Immunol. 2015, 26, 291–294. [Google Scholar] [CrossRef]
- Fernandez-Rivas, M.; Vereda, A.; Vickery, B.P.; Sharma, V.; Nilsson, C.; Muraro, A.; Hourihane, J.O.; DunnGalvin, A.; du Toit, G.; Blumchen, K.; et al. Open-Label Follow-on Study Evaluating the Efficacy, Safety, and Quality of Life with Extended Daily Oral Immunotherapy in Children with Peanut Allergy. Allergy 2022, 77, 991–1003. [Google Scholar] [CrossRef]
- Saleh-Langenberg, J.; Flokstra-de Blok, B.M.J.; Goossens, N.J.; Kemna, J.C.; van der Velde, J.L.; Dubois, A.E.J. The Compliance and Burden of Treatment with the Epinephrine Auto-Injector in Food-Allergic Adolescents. Pediatr. Allergy Immunol. 2016, 27, 28–34. [Google Scholar] [CrossRef]
- van der Valk, J.P.M.; Gerth van Wijk, R.; Flokstra-de Blok, B.M.J.; van der Velde, J.L.; de Groot, H.; Wichers, H.J.; Dubois, A.E.J.; de Jong, N.W. No Difference in Health-Related Quality of Life, after a Food Challenge with Cashew Nut in Children Participating in a Clinical Trial. Pediatr. Allergy Immunol. 2016, 27, 812–817. [Google Scholar] [CrossRef]
- de Weger, W.W.; Kunst, M.; Herpertz, C.E.M.; van der Meulen, G.; van Lente, L.; Koppelman, G.H.; Sprikkelman, A.B.; Kamps, A.W.A. Low Health-Related Quality of Life Is Associated with Declining Home Introduction of Suspected Food Allergens. Clin. Exp. Allergy 2022, 52, 201–204. [Google Scholar] [CrossRef]
- Blumchen, K.; Trendelenburg, V.; Ahrens, F.; Gruebl, A.; Hamelmann, E.; Hansen, G.; Heinzmann, A.; Nemat, K.; Holzhauser, T.; Roeder, M.; et al. Efficacy, Safety, and Quality of Life in a Multicenter, Randomized, Placebo-Controlled Trial of Low-Dose Peanut Oral Immunotherapy in Children with Peanut Allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 479–491.e10. [Google Scholar] [CrossRef] [Green Version]
- Frachette, C.; Fina, A.; Fontas, E.; Donzeau, D.; Hoflack, M.; Gastaud, F.; Baechler, E.; Dor, E.; Descos, B.; Triolo, V.; et al. Health-Related Quality of Life of Food-Allergic Children Compared with Healthy Controls and Other Diseases. Pediatr. Allergy Immunol. 2022, 33, e13663. [Google Scholar] [CrossRef]
- Acaster, S.; Gallop, K.; de Vries, J.; Ryan, R.; Vereda, A.; Knibb, R.C. Peanut Allergy Impact on Productivity and Quality of Life (PAPRIQUA): Caregiver-Reported Psychosocial Impact of Peanut Allergy on Children. Clin. Exp. Allergy 2020, 50, 1249–1257. [Google Scholar] [CrossRef]
- Reier-Nilsen, T.; Carlsen, K.C.L.; Michelsen, M.M.; Drottning, S.; Carlsen, K.-H.; Zhang, C.; Borres, M.P.; Håland, G. Parent and Child Perception of Quality of Life in a Randomized Controlled Peanut Oral Immunotherapy Trial. Pediatr. Allergy Immunol. 2019, 30, 638–645. [Google Scholar] [CrossRef]
- Morou, Z.; Vassilopoulou, E.; Galanis, P.; Tatsioni, A.; Papadopoulos, N.G.; Dimoliatis, I.D.K. Investigation of Quality of Life Determinants in Children with Food Allergies. Int. Arch. Allergy Immunol. 2021, 182, 1058–1065. [Google Scholar] [CrossRef]
- O’B Hourihane, J.; Beyer, K.; Abbas, A.; Fernández-Rivas, M.; Turner, P.J.; Blumchen, K.; Nilsson, C.; Ibáñez, M.D.; Deschildre, A.; Muraro, A.; et al. Efficacy and Safety of Oral Immunotherapy with AR101 in European Children with a Peanut Allergy (ARTEMIS): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 3 Trial. Lancet Child Adolesc. Health 2020, 4, 728–739. [Google Scholar] [CrossRef]
- Miller, J.; Blackman, A.C.; Wang, H.T.; Anvari, S.; Joseph, M.; Davis, C.M.; Staggers, K.A.; Anagnostou, A. Quality of Life in Food Allergic Children: Results from 174 Quality-of-Life Patient Questionnaires. Ann. Allergy Asthma Immunol. 2020, 124, 379–384. [Google Scholar] [CrossRef]
- Dantzer, J.A.; Wood, R.A. The Impact of Tree Nut Oral Food Challenges on Quality of Life and Acute Reactions in Nut Allergic Patients. J. Allergy Clin. Immunol. Pract. 2019, 7, 698–700.e1. [Google Scholar] [CrossRef]
- DunnGalvin, A.; Koman, E.; Raver, E.; Frome, H.; Adams, M.; Keena, A.; Hourihane, J.O.; Gallagher, P.L.; Blok, B.F.; Dubois, A.; et al. An Examination of the Food Allergy Quality of Life Questionnaire Performance in a Countrywide American Sample of Children: Cross-Cultural Differences in Age and Impact in the United States and Europe. J. Allergy Clin. Immunol. Pract. 2017, 5, 363–368.e2. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Hass, S.L.; Donelson, S.M.; Robison, D.; Cameron, A.; Etschmaier, M.; Duhig, A.; McCann, W.A. The Peanut Allergy Burden Study: Impact on the Quality of Life of Patients and Caregivers. World Allergy Organ. J. 2021, 14, 100512. [Google Scholar] [CrossRef]
- Soller, L.; Clarke, A.E.; Lyttle, A.; Chin, R.; Ben-Shoshan, M.; Cheuk, S.; Asai, Y.; Chan, E.S. Comparing Quality of Life in Canadian Children with Peanut, Sesame, and Seafood Allergy. J. Allergy Clin. Immunol. Pract. 2020, 8, 352–354.e1. [Google Scholar] [CrossRef]
- Epstein-Rigbi, N.; Goldberg, M.R.; Levy, M.B.; Nachshon, L.; Elizur, A. Quality of Life of Food-Allergic Patients Before, During, and After Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2019, 7, 429–436.e2. [Google Scholar] [CrossRef]
- Epstein Rigbi, N.; Schwartz, N.; Goldberg, M.R.; Levy, M.B.; Nachshon, L.; Elizur, A. Medical Clown Support Is Associated with Better Quality of Life of Children with Food Allergy Starting Oral Immunotherapy. Pediatr. Allergy Immunol. 2021, 32, 1029–1037. [Google Scholar] [CrossRef]
- DunnGalvin, A.; Treneva, M.; Pampura, A.; Grebenko, A.; Makatsori, M.; Munblit, D. Quality of Life Associated with Maternal Anxiety Disorder in Russian Children and Adolescents with Food Allergy. Pediatr. Allergy Immunol. 2019, 31, 78–84. [Google Scholar] [CrossRef]
- Arik Yilmaz, E.; Cavkaytar, O.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sekerel, B.E.; DunnGalvin, A.; Karabulut, E.; Sackesen, C. Factors Affecting Food Allergy-Related Quality of Life From Parents’ Perception in Turkish Children. Allergy Asthma Immunol. Res. 2018, 10, 379–386. [Google Scholar] [CrossRef]
- Mizuno, Y.; Ohya, Y.; Nagao, M.; DunnGalvin, A.; Fujisawa, T. Validation and Reliability of the Japanese Version of the Food Allergy Quality of Life Questionnaire–Parent Form. Allergol. Int. 2017, 66, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Golding, M.A.; Batac, A.L.R.; Gunnarsson, N.V.; Ahlstedt, S.; Middelveld, R.; Protudjer, J.L.P. The Burden of Food Allergy on Children and Teens: A Systematic Review. Pediatr. Allergy Immunol. 2022, 33, e13743. [Google Scholar] [CrossRef]

| Search String |
|---|
| (“food allergy” OR “food allergies”) AND “quality of life” AND (child OR children OR adolescent OR adolescents OR adolescence OR teen OR teenager OR teenagers) |
| Participants | Intervention | Comparison | Outcomes | Study Design | |
|---|---|---|---|---|---|
| Observational studies | Children and adolescents 6–18 years old, or/and their parents | Food Allergy diagnosis | Between food allergy patients and healthy population | Quality of Life or Health-Related Quality of Life | Cross-Sectional, Cohort and Case-Control Studies |
| Interventional studies | Children and adolescents 6–18 years old, or/and their parents | Oral Food Challenge or Oral Immunotherapy | Before and after the intervention or between groups that underwent or not an intervention | Quality of Life or Health-Related Quality of Life | Clinical Trials |
| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
| First Author | Country/Region | Study Design | Study Population and FA | Sample Size | Participant Age (Years) | FA Diagnosis | |
|---|---|---|---|---|---|---|---|
| Miller [61] | USA | Cross- Sectional | Adolescents (13–17 y) and parents (as proxies) of children (0–12 y) with FA in peanuts, nuts, milk, egg, wheat, soya, sesame, fish, shellfish, fruit, vegetables or other foods | Teens: 24 | ND | physician-diagnosed | |
| Parents: 150 | |||||||
| Dunn Galvin [68] | Russia | Cross- Sectional | Children (7–12 y), adolescents (13–17 y) and parents (as proxies) of children 7–12 y with FA in peanuts, milk, egg, hazelnut, almond, walnut, sesame, fish, shellfish, fruit, or other foods | Children: 44 | 9.9 ± 4.8 | parent-and/or self-reported clinical history and SPT/specific IgE | |
| Teens: 48 | |||||||
| Parents: 44 | |||||||
| Protudjer [45] | Sweden | Cross-Sectional | Adolescents (13–17 y) with FA in cow’s milk, hen’s egg, or wheat | 57 | ND | history of FA and positive OFC or high food-specific IgE | |
| Dantzer [62] | USA | Cross- Sectional | Children (8–12 y), adolescents (13–18 y) and parents (as proxies) of children <8 y with FA who underwent OFC in the past 2 years, but were still avoiding ≥1 tree nut/peanut, or declined OFC and were avoiding all nuts | Children: n = 18 Teens: n = 10 Parents: n = 58 | 9.7 | history of tree-nut allergy and positive SPT or high food-specific IgE | |
| Manso [49] | Spain | Cross- Sectional | Parents (as proxies) of children 7–12 y with FA in eggs, nuts (including peanut), milk, fish/shellfish, fruits or other foods | N = 54 | ND | positive OFC and positive SPT or high food-specific IgE | |
| Dunn Galvin [63] | USA | Cross- Sectional | Parents (as proxies) of children 0–12 y with FA (specific FA’s not reported) | N = 1029 | ND | physician-diagnosed | |
| Stensgaard [37] | Denmark | Cross- Sectional | Children (8–12 y) and adolescents (13–17 y) with FA in peanuts, nuts eggs, hazelnuts or other foods, and their parents (as proxies) | Children: n = 73 Teens: n = 49 Parents: n = 143 | Children: 10.33 ± 1.4 | positive OFC and positive SPT or high food-specific IgE | |
| Teens: 14.94± 1.4 | |||||||
| Protudjer [46] | Sweden | Cross- Sectional | Adolescents (13–17 y) with FA in cow’s milk, hen’s egg and/or wheat | N = 58 | ND | physician-diagnosed | |
| Morou [59] | Greece | Cross- Sectional | Children (8–12 y) with FA in nuts, fish, egg, legumes, milk, cereal, shellfish, fruit, meat, dark chocolate, spices or food supplements | N = 110 | 10.0 ± 1.4 | physician-diagnosed | |
| Nowak-Wegrzyn [64] | USA | Cross- Sectional | Adolescents (13–17 y) with peanut FA | N = 102 | 14.6 ± 1.3 | physician-diagnosed | |
| Yilmaz [69] | Turkey | Cross- Sectional | Parents (as proxies) of children (7–12 y) with FA in cow’s milk, egg, hazelnut, walnut, peanut, legume, pistachio, wheat, sesame, meat, fish, cashew, pumpkin seeds, or banana | N = 25 | 9.3 (7.8–11.4) | positive SPT or high food-specific IgE and positive OFC, or a clear-cut history of anaphylaxis with food | |
| Acaster [57] | U.K. | Cross- Sectional | Parents (as proxies) of children (4–15 y) with peanut FA | N = 100 | 9.82 ± 3.42 | physician-diagnosed | |
| Soller [65] | Canada | Cross- Sectional | Parents (as proxies) of children with peanut, sesame or seafood FA | N = 793 | 9.32 (6.91, 11.37) | physician-diagnosed | |
| Thörnqvist [39] | Sweden | Cross- Sectional | Parents (as proxies) of children (0–12 y) with FA in hen’s egg, tree nuts, peanuts, or other foods | N = 63 | ND | history of FA to ≥1 food and a positive ImmunoCAP test for allergen-specific IgE antibodies to the same food | |
| Saleh-Langenberg [52] | The Netherlands | Cross- Sectional | Adolescents (13–17 y) with FA in tree nuts, peanuts, fruit, soy, milk, vegetables, shellfish, sesame, wheat, fish, or celery, who were prescribed an EAI and parents (as proxies) | N = 55 | 15.9 ± 1.29 | physician-diagnosed | |
| Mizuno [70] | Japan | Case- Control | Parents (as proxies) of children (0–12 y) with and without FA in egg, milk, peanut, wheat, or other foods | Cases: n = 25 Controls: n = 17 | ND | physician-diagnosed | |
| Strinnholm [47] | Sweden | Case- Control | Adolescents (12–13 y) with and without food hypersensitivity in milk, egg, cod, or wheat | Cases: n = 74 Controls: n = 209 | ND | clinical examination including a structured interview, high specific IgE to the culprit food and a celiac screen test | |
| Protudjer [48] | Sweden | Case- Control | Parents (as proxies) of children (0–12 y) with FA in hen’s egg, wheat, or milk, and without FA | Cases: n = 85 Controls: n = 94 | 6.0 | physician-diagnosed and history of FA to ≥1 food (cow’s milk, hen’s egg and/or wheat) as ascertained either by a positive OFC or by high levels of food-specific IgE | |
| Frachette [56] | France | Case- Control | Children (8–12 y) and adolescents (13–17 y) with FA (in peanuts, nuts, eggs, cow’s milk, kiwi, fish, goat’s milk, mustard, pine nuts, crustaceans, legumes, rosacea, wheat, soya or other foods), vs. healthy controls and children with other diagnoses | Cases: n = 135 Controls: n = 500 | 11.6 ± 2.49 | history of FA, physical examinations, blood tests and SPT | |
| Epstein-Rigbi [66] | Israel | Cohort | Parents (as proxies) of children (4–12 y) with FA (in milk, peanut, egg, sesame, or tree nuts) who undergo OΙΤ vs. controls | N = 223 | OIT: 6.3 ± 2.3 Controls: 6.8 ± 2.3 | positive OFC and positive SPT or high food-specific IgE | |
| Epstein Rigbi [67] | Israel | Cohort | Children (8–12 y) with FA (in milk, peanut, egg, sesame, or tree nuts) who underwent OIT, vs. controls | N = 103 | 9.0 (8.0–11.0) | positive SPT and/or high specific serum IgE, and positive OFC or clinical history of allergic reaction in the past year | |
| Vazquez-Ortiz [50] | Spain | Cohort | Children (8–12 y) with FA who underwent egg OIT | N = 18 | 9.1 ± 1.3 | physician-diagnosed egg FA | |
| de Weger [54] | The Netherlands | Cohort | Children (0–12 y) and parents (as proxies) of children with FA, recommended to introduce peanut/tree nut at home | Children: n = 19 Parents: n = 23 | ND | physician-diagnosed | |
| First Author | Country/Region | Study Design | Study Population | Time of Assessment of HRQoL | Sample Size | Participant Age (Years) * | FA Definition |
|---|---|---|---|---|---|---|---|
| Reier-Nilsen [58] | Norway | Clinical trial | Children (5–15 y) with sensitization to peanut who underwent OIT vs. controls | at enrollment, after 1 year and after 2 years of OIT | N = 77 | 9.3 | sensitization to peanut by a positive peanut SPT and/or high peanut-specific IgE or history of systemic reactions to peanuts |
| van der Valk [53] | The Netherlands | Clinical trial | Children (8–12 y), adolescents (13–17 y) and parents (as proxies) of children 2–12 y who underwent double-blind, placebo-controlled food challenges with cashew nut | before the challenge and 6 months after | Children: n = 33 Teens: n = 26 Parents: n = 84 | 9.0 | history of FA and positive SPT or high food-specific IgE |
| Fernandez-Rivas [51] | Spain | Clinical trial | Children and adolescents (4–17 y) with FA in peanuts (and their parents as proxies), who underwent OIT, vs. a placebo group | at baseline, after 1 year and after 1.5 or 2 years | N = 142 | 10.0 (7.0–12.0) | clinical history of FA to peanuts, positive serum IgE to peanut, immunoCAP, and/or a positive SPT to peanut |
| Hourihane [60] | European | MC, DB, randomized, placebo-controlled trial | Children and adolescents (4–17 y) with FA in peanuts who underwent OIT, vs. a placebo group | before OIT and at the end of trial | N = 175 | 9.1 ± 3.7 | clinical history, positive SPT, high food-specific IgE, and OFC |
| Blumchen [55] | Germany | MC, DB, randomized placebo-controlled trial | Children and adolescents (3–17 y) with peanut allergy who underwent OIT, vs. a placebo arm | 4 weeks before the initial OFC and 4 weeks post-final OFC | N = 62 | 6.6 (4.8–9.8) | high serum peanut-specific IgE, and challenge-proven clinically relevant PA |
| First Author | Instrument | Population | Domain, Score Range [Worst, Best] | QoL Score | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Miller [61] | FAQLQ-PF FAQLQ-TF | Children (parent-proxy) and teens with FA | Children † | Adolescents † | Children vs. Adolescents | ||||
| Emotional impact [7, 1] | 3.1 (1.0–6.8) | 3.8 (1.8–6.3) | 0.02 | ||||||
| Food anxiety [7, 1] | 3.8 (1.0–7.5) | ΝA | ΝA | ||||||
| Social and dietary limitations [7, 1] | 4 (1.0–7.0) | 5.2 (2.3–7.0) | 0.002 | ||||||
| Total QoL [7, 1] | 3.5 (1.1–6.9) | 4.7 (1.9–6.8) | 0.007 | ||||||
| FAIM | 3 (0.4–5.0) | 2.7 (0.6–4.7) | 0.78 | ||||||
| Dunn Galvin [68] | FAQLQ-PF FAQLQ-CF FAQLQ-TF | Children, parents (as proxies) and teens with FA | |||||||
| Parents ‡ | Children ‡ | Adolescents ‡ | |||||||
| Total QoL [7, 1] | 3.6 ± 1.3 | 3.9 ± 1.1 | 3.8 ± 1.7 | ΝA | |||||
| FAIM | 3.7 ± 0.7 | 3.8 ± 0.8 | 3.6 ± 0.9 | ΝA | |||||
| Protudjer [45] | FAQLQ-TF | Teens with FA | Boys M | Girls M | Total M | Boys vs. Girls | |||
| Allergen avoidance and dietary restrictions [7, 1] | |||||||||
| 5.14 | 5.49 | 5.25 | ΝD | ||||||
| Emotional impact, [7, 1] | 4.35 | 5.30 | 4.65 | <0.01 | |||||
| Risk of accidental exposure [7, 1] | 4.46 | 4.42 | 4.45 | ΝD | |||||
| Total QoL [7, 1] | 4.81 | 5.29 | 4.96 | ΝD | |||||
| Dantzer [62] | FAQLQ-PF | Parents (as proxies) of children with nut FA who underwent OFC, or not | with OFC M | without OFC M | |||||
| Emotional impact [7, 1] | 3.25 | 3.38 | ΝD | ||||||
| Food anxiety [7, 1] | 3.71 | 3.81 | ΝD | ||||||
| Social and dietary limitations [7, 1] | 3.5 | 3.72 | ΝD | ||||||
| Total QoL [7, 1] | 3.45 | 3.61 | ΝD | ||||||
| FAQLQ-CF | Children with nut allergy who underwent OFC, or not | Emotional impact [7, 1] | 5.04 | 4.25 | ΝD | ||||
| Allergen avoidance [7, 1] | 4.88 | 4.21 | ΝD | ||||||
| Risk of accidental exposure [7, 1] | 4.67 | 4.33 | ΝD | ||||||
| Total QoL [7, 1] | 4.83 | 4.30 | ΝD | ||||||
| FAQLQ-TF | Adolescents with nut allergy who underwent OFC, or not | Emotional impact [7, 1] | 3.48 | 5.04 | ΝD | ||||
| Allergen avoidance [7, 1] | 3.86 | 3.97 | ΝD | ||||||
| Risk of accidental exposure [7, 1] | 3.86 | 4.05 | ΝD | ||||||
| Total QoL [7, 1] | 3.74 | 4.44 | ΝD | ||||||
| Manso [49] | FAQLQ-PF | Parents of children with FA | |||||||
| Emotional impact [7, 1] | 2.9 ± 1.0 ‡ | ||||||||
| Food anxiety [7, 1] | 3.4 ± 1.5 ‡ | ||||||||
| Social and dietary limitations [7, 1] | 2.6 ± 1.2 ‡ | ||||||||
| Total QoL [7, 1] | 3.0 ± 1.1 ‡ | ||||||||
| Dunn Galvin [63] | FAQLQ-PF | Boys M | Girls M | USA M | Europe M | ||||
| Parents (as proxies) of children with FA | |||||||||
| Emotional impact [7, 1] | 3.91 | 4.25 | 4.06 | ΝD | ΝD | ||||
| Food anxiety [7, 1] | 4.27 | 4.63 | 4.42 | ΝD | ΝD | ||||
| Social and dietary limitations [7, 1] | 4.29 | 4.52 | 4.39 | ΝD | ΝD | ||||
| Total QoL [7, 1] | 4.16 | 4.45 | 4.29 | 3.8 | ΝD | ||||
| Stensgaard [37] | FAQLQ-CF | Boys ‡ | Girls ‡ | ||||||
| Children with peanut, hazelnut or egg FA | Allergen avoidance [7, 1] | 3.56±1.50 | 3.84±1.58 | ΝD | |||||
| Dietary restrictions [7, 1] | 3.49±1.53 | 4.15±1.54 | ΝD | ||||||
| Emotional impact [7, 1] | 4.10 ± 1.68 | 4.52 ± 1.60 | ΝD | ||||||
| Risk of accidental exposure [7, 1] | 3.38 ± 1.55 | 3.99 ± 1.76 | ΝD | ||||||
| Total QoL [7, 1] | 3.64 ± 1.39 | 4.12 ± 1.51 | ΝD | ||||||
| FAIM | 3.08 ± 1.16 | 3.82 ± 1.39 | ΝD | ||||||
| FAQLQ-TF | Adolescents peanut, hazelnut or egg FA | Allergen avoidance and dietary restrictions [7, 1] | 3.66 ± 1.62 | 4.39 ± 1.21 | ΝD | ||||
| Emotional impact [7, 1] | 3.90 ± 1.47 | 4.46 ± 1.21 | ΝD | ||||||
| Risk of accidental exposure [7, 1] | 3.59 ± 1.69 | 4.03 ± 1.66 | ΝD | ||||||
| Total QoL [7, 1] | 3.71 ± 1.51 | 4.32 ± 1.20 | ΝD | ||||||
| FAIM | 3.42 ± 1.06 | 3.45 ± 1.22 | ΝD | ||||||
| FAQLQ-PF | Parents (as proxies) of children with peanut, hazelnut or egg FA | Fathers ‡ | Mothers ‡ | ||||||
| Emotional impact [7, 1] | 2.76 ± 1.06 | 2.85 ± 1.21 | ΝD | ||||||
| Food anxiety [7, 1] | 3.24 ± 1.30 | 3.26 ± 1.38 | ΝD | ||||||
| Social and dietary limitations [7, 1] | 2.65 ± 1.30 | 2.57 ± 1.31 | ΝD | ||||||
| Total QoL [7, 1] | 2.89 ± 1.14 | 2.89 ± 1.20 | ΝD | ||||||
| FAIM | 3.89 ± 0.84 | 4.01 ± 0.89 | ΝD | ||||||
| FAQLQ-PF | Parents (as proxies) of adolescents with peanut, hazelnut or egg FA | Emotional impact [7, 1] | 3.04 ± 1.62 | 3.18 ± 1.25 | ΝD | ||||
| Food anxiety [7, 1] | 3.30 ± 1.31 | 3.61 ± 1.41 | ΝD | ||||||
| Social and dietary limitations [7, 1] | 2.43±1.13 | 2.97±1.49 | ΝD | ||||||
| Total QoL [7, 1] | 2.92±1.13 | 3.25±1.32 | ΝD | ||||||
| FAIM | 3.97±0.58 | 4.00±0.89 | ΝD | ||||||
| Protudjer [46] | FAQLQ-TF | Boys ‡ | Girls ‡ | Total M | Boys vs. girls | ||||
| Adolescents with staple FA | Allergen avoidance and dietary restrictions [7, 1] | ||||||||
| ND | ND | 4.95 | ND | ||||||
| Risk of accidental exposure [7, 1] | ND | ND | 4.19 | ND | |||||
| Emotional impact [7, 1] | 4.50±0.24 | 5.38±1.4 | ΝD | 0.04 | |||||
| Total QoL [7, 1] | 4.51±1.23 | 5.12±1.01 | 4.70 | 0.07 | |||||
| Morou [59] | FAQLQ-CF | Total ‡ | |||||||
| Children with FA | Emotional impact [7, 1] | 3.98 ± 1.21 | |||||||
| Allergen avoidance [7, 1] | 2.45 ± 1.26 | ||||||||
| Risk of accidental exposure [7, 1] | 2.69 ± 1.27 | ||||||||
| Dietary restrictions [7, 1] | 2.55 ± 1.30 | ||||||||
| Total QoL [7, 1] | 2.92 ± 1.08 | ||||||||
| FAIM | 2.95 ± 1.06 | ||||||||
| PedsQL 4.0 | Physical functioning [100, 0] | 91.42 ± 10.99 | |||||||
| Emotional functioning [100, 0] | 81.68 ± 17.86 | ||||||||
| Social functioning [100, 0] | 87.31 ± 16.76 | ||||||||
| School functioning [100, 0] | 89.59 ± 13.05 | ||||||||
| Total QoL [100, 0] | 88.01 ± 11.22 | ||||||||
| Nowak-Wegrzyn [64] | FAQLQ-TF | Total ‡ | |||||||
| Adolescents with peanut FA | Emotional impact [7, 1] | 4.9 ± 1.3 | |||||||
| Allergen avoidance [7, 1] | 5.0 ± 1.3 | ||||||||
| Risk of accidental exposure [7, 1] | 5.0 ± 1.3 | ||||||||
| Total QoL [7, 1] | 5.0 ± 1.8 | ||||||||
| FAIM | 4.3 ± 1.2 | ||||||||
| PedsQL 4.0 | vs. Healthy | vs. other diagnoses | |||||||
| Adolescents with peanut FA | Physical functioning [0, 100] | 75.4 ± 29.4 | <0.001 | 0.180 | |||||
| Psychosocial health [0, 100] | 66.2 ± 23.4 | <0.001 | 0.035 | ||||||
| Emotional functioning [0, 100] | 61.3 ± 26.7 | <0.001 | 0.004 | ||||||
| Social functioning [0, 100] | 69.6 ± 27.7 | <0.001 | 0.021 | ||||||
| School functioning [0, 100] | 69.6 ± 27.7 | <0.001 | 0.804 | ||||||
| Total QoL [0, 100] | 69.4 ± 23.0 | <0.001 | 0.045 | ||||||
| Yilmaz [69] | FAQLQ-PF | Parents(as proxies) of children with FAs | Total ƒ | ||||||
| Emotional impact [7, 1] | 3.1 (0.3) | ||||||||
| Food anxiety [7, 1] | 3.9 (0.3) | ||||||||
| Social and dietary limitations [7, 1] | 2.9 (0.3) | ||||||||
| Total QoL [7, 1] | 3.3 (0.3) | ||||||||
| Acaster [57] | FAQLQ-PF | Parents (as proxies) of children with peanut FA | |||||||
| Emotional impact [7, 1] | 3.14 ± 1.60 | ||||||||
| Food anxiety [7, 1] | 3.72 ± 1.65 | ||||||||
| Social and dietary limitations [7, 1] | 3.40 ± 1.63 | ||||||||
| Total QoL [7, 1] | 3.37 ± 1.57 | ||||||||
| FAIM | 3.78 ± 0.89 | ||||||||
| EQ-5D | Total QoL [1, 0] | 0.873 ± 0.231 | |||||||
| Soller [65] | FAQLQ-PF10 | Parents (as proxies) of children with peanut, sesame and seafood FA | |||||||
| Total QoL of all patients | 2.50 ± 1.37 | ||||||||
| Total QoL of peanut FA patients | 2.53 ± 1.34 | ||||||||
| Total QoL of sesame FA patients | 2.56 ± 1.53 | ||||||||
| Total QoL of seafood FA patients | 1.97 ± 1.63 | ||||||||
| Thörnqvist [39] | FAQLQ-PF | Parents (as proxies) of children with FAs | |||||||
| Emotional impact [7, 1] | 2.56 ± 1.35 | ||||||||
| Food anxiety [7, 1] | 2.48 ± 1.38 | ||||||||
| Social and dietary limitations [7, 1] | 2.89 ± 1.56 | ||||||||
| Total QoL [7, 1] | 2.65 ± 1.32 | ||||||||
| Saleh-Langenberg [52] | FAQLQ-TF | Adolescents with FAs who had been prescribed an EAI | Allergen avoidance [7, 1] | 4.02 ± 1.44 | |||||
| Risk of accidental exposure [7, 1] | 3.92 ± 1.46 | ||||||||
| Emotional impact [7, 1] | 3.99 ± 1.51 | ||||||||
| Total QoL [7, 1] | 4.03 ± 1.35 | ||||||||
| FAIM | 3.57 ± 0.96 | ||||||||
| FAQLQ-PF | Parents of adolescents who had been prescribed an EAI | Emotional impact [7, 1] | 2.82 ± 1.02 | NA | NA | ||||
| Food anxiety [7, 1] | 3.83 ± 1.08 | NA | NA | ||||||
| Social restrictions [7, 1] | 2.82 ± 1.02 | NA | NA | ||||||
| Dietary restrictions [7, 1] | 3.85 ± 1.32 | NA | NA | ||||||
| Total QoL [7, 1] | 3.42 ± 0.97 | NA | NA | ||||||
| Mizuno [70] | FAQLQ-PF | Parents (as proxies) of children with FA and controls | with FA ‡ | Controls ‡ | With FAs vs. no FAs | ||||
| Emotional impact [7, 1] | 3.6 ± 1.4 | 0.4 ± 0.9 | <0.001 | ||||||
| Food anxiety [7, 1] | 4.3 ± 1.6 | 0.4 ± 0.9 | <0.001 | ||||||
| Social and dietary limitations [7, 1] | 4.0 ± 1.5 | 0.4 ± 0.9 | <0.001 | ||||||
| Total QoL [7, 1] | 3.8 ± 1.3 | 0.4 ± 0.8 | <0.001 | ||||||
| Strinnholm [47] | FAQLQ-ΤF | Allergen avoidance and dietary restrictions [7, 1] | Boys M | Girls M | Total M | Boys vs. girls | |||
| Adolescents with FH | 3.57 | 3.75 | 3.67 | 0.579 | |||||
| Emotional impact [7, 1] | 2.78 | 2.90 | 2.86 | 0.711 | |||||
| Risk of accidental exposure [7, 1] | 3.66 | 3.97 | 3.84 | 0.324 | |||||
| Total QoL [7, 1] | 3.40 | 3.60 | 3.51 | 0.496 | |||||
| Girls | Boys | Girls | Boys | ||||||
| KIDSCREEN-52 | FH vs. controls | ||||||||
| with FHŠ | ControlsŠ | with FHŠ | ControlsŠ | ||||||
| Adolescents with FH vs. controls | Physical Well-being | 49.6 | 49.6 | 49.6 | 49.6 | 0.641 | 0.521 | ||
| Psychological Well-being | 51.8 | 51.7 | 51.8 | 54.5 | 0.447 | 0.172 | |||
| Moods and Emotions | 50.2 | 54.0 | 54.0 | 55.7 | 0.702 | 0.982 | |||
| Self-Perception | 49.8 | 52.2 | 52.2 | 55.4 | 0.879 | 0.199 | |||
| Autonomy | 48.7 | 50.7 | 53.2 | 53.2 | 0.879 | 0.646 | |||
| Parent Relation and Home Life | 54.6 | 54.6 | 54.6 | 54.6 | 0.691 | 0.759 | |||
| Financial Resources | 56.3 | 56.3 | 56.3 | 56.3 | 0.945 | 0.942 | |||
| Social Support and Peers | 52.4 | 54.9 | 48.3 | 50.2 | 0.667 | 0.828 | |||
| School Environment | 54.2 | 54.2 | 52.2 | 52.2 | 0.905 | 0.660 | |||
| Social Acceptance and Bullying | 58.8 | 58.8 | 58.8 | 58.8 | 0.037 | 0.947 | |||
| Protudjer [48] | FAQLQ-PF | Parents (as proxies) of children with FA | Emotional impact [7, 1] | ~2.9 M | |||||
| Food anxiety [7, 1] | ~3 M | ||||||||
| Social and dietary limitations [7, 1] | ~3.3 M | ||||||||
| Total QoL [7, 1] | ~3.1 M | ||||||||
| EQ-5D | Parents (as proxies) of children with FA vs. controls | with FA M | Controls M | ||||||
| Total QoL [1, 0] | 0.84 M | 0.94 | <0.01 | ||||||
| Epstein Rigbi [67] | OIT group | controls | baseline vs. 6 months post-OIT/controls | ||||||
| mean change (95% CI) post-OIT | |||||||||
| FAQLQ-CF | Children who underwent OIT vs. controls | Emotional impact [7, 1] | −1.1 (−2.5, 0.0) | −0.3 (−0.9, 0.8) | <0.001/0.44 | ||||
| Allergen avoidance [7, 1] | −1.3 (−2.1, −0.2) | 0.0 (−1.5, 0.9) | <0.001/0.64 | ||||||
| Dietary restrictions [7, 1] | −0.7 (−2.5, 0.5) | −0.5 (−1.6, 0.4) | 0.008/0.06 | ||||||
| Risk of accidental exposure [7, 1] | −0.9 (−2.9, −0.4) | 0.0 (−1.2, 0.6) | <0.001/0.44 | ||||||
| Total QoL [7, 1] | −1.0 (−2.3, −0.3) | −0.2 (−0.9, 0.4) | <0.001/0.13 | ||||||
| pre-OIT | post-IOT | Pre-vs. post-OIT | |||||||
| FAQLQ-PF | Parents (as proxies) of children who underwent OIT before and after OIT | ||||||||
| Emotional impact [7, 1] | 4.2 (3.1–4.8) m | 2.5 (1.8–3.6) m | <0.001 | ||||||
| Food anxiety [7, 1] | 4.4 (3.1–5.8) m | 2.4 (1.4–3.6) m | <0.001 | ||||||
| Social and dietary limitations [7, 1] | 4.0 (2.1–5.0) m | 1.7 (1.0–3.2) m | <0.001 | ||||||
| Total QoL [7, 1] | 4.0 (3.2–5.0) m | 2.2 (1.6–3.6) m | <0.001 | ||||||
| Children‡ | Adolescents‡ | ||||||||
| Frachette [56] | FAQLQ-CF FAQLQ-TF | Children and teens with FA | |||||||
| Allergen avoidance [7, 1] | 3.40 ± 1.65 | 3.83 ± 1.44 | NA | ||||||
| Risk of accidental exposure [7, 1] | 3.59 ± 1.55 | 3.39 ± 1.49 | NA | ||||||
| Emotional impact [7, 1] | 4.74 ± 1.51 | 3.74 ± 1.43 | NA | ||||||
| Dietary restrictions [7, 1] | 3.96 ± 1.73 | ND | NA | ||||||
| Total QoL [7, 1] | 3.91 ± 1.44 | 3.69 ± 1.27 | NA | ||||||
| FAIM | 3.33 ± 1.14 | 3.32 ± 0.98 | NA | ||||||
| CHQ-CF87 | Children with FAs vs. controls | with FA‡ | Controls‡ | ||||||
| Behavior [0, 100] | 84.49 ± 9.63 | 83.75 ± 12.36 | ND | ||||||
| Bodily Pain [0, 100] | 79.45 ± 19.8 | 70.17 ± 23 | ND | ||||||
| Family activities [0, 100] | 91.32 ± 13.26 | 87.89 ± 17.5 | ND | ||||||
| Family cohesion [0, 100] | 79.23 ± 20.83 | 77.65 ± 24.66 | ND | ||||||
| General health perception [0, 100] | 73.64 ± 15.84 | 75.62 ± 16.09 | ND | ||||||
| Mental health [0, 100] | 80.96 ± 11.42 | 75.18 ± 15.61 | ND | ||||||
| Physical functioning [0, 100] | 90.56 ± 20.05 | 93.18 ± 14.17 | ND | ||||||
| Role/Social limitations-Behavioral [0, 100] | 93.89 ± 14.84 | 94.74 ± 12.8 | ND | ||||||
| Role/Social limitations-Emotional [0, 100] | 90.96 ± 17.36 | 92.31 ± 15.85 | ND | ||||||
| Role/Social limitations-Physical [0, 100] | 93.89 ± 15.82 | 94.44 ± 12.61 | ND | ||||||
| Self-esteem [0, 100] | 84.95 ± 12.09 | 83.09 ± 15.3 | ND | ||||||
| Adolescents with FAs vs. controls | with FA‡ | controls‡ | |||||||
| Behavior [0, 100] | 85.33 ± 11.74 | 79.72 ± 12.94 | ND | ||||||
| Bodily Pain [0, 100] | 74.77 ± 26.19 | 67.95 ± 23.02 | ND | ||||||
| Family activities [0, 100] | 91.7 ± 13.06 | 86.62 ± 17.92 | ND | ||||||
| Family cohesion [0, 100] | 79.43 ± 21.76 | 70.84 ± 25.7 | ND | ||||||
| General health perception [0, 100] | 67.98 ± 16.89 | 73.5 ± 15.17 | ND | ||||||
| Mental health [0, 100] | 78.76 ± 14.52 | 73.08 ± 14.95 | ND | ||||||
| Physical functioning [0, 100] | 96.13 ± 5.36 | 94.43 ± 14.09 | ND | ||||||
| Role/Social limitations-Behavioral [0, 100] | 97.22 ± 10.2 | 91.99 ± 16.35 | ND | ||||||
| Role/Social limitations-Emotional [0, 100] | 94.7 ± 12.79 | 89.16 ± 12.79 | ND | ||||||
| Role/Social limitations-Physical [0, 100] | 97.22 ± 11.27 | 94.82 ± 15.22 | ND | ||||||
| Self-esteem [0, 100] | 77.6 ± 16.52 | 74.86 ± 13.81 | ND | ||||||
| OIT group | Control group | Pre-vs. post-OIT | |||||||
| Epstein-Rigbi [66] | FAQLQ-PF | Parents (as proxies) of children with FA before and at 6 months post-OIT vs. controls | pre-OIT M | post-OIT M | pre-OIT M | post-OIT M | |||
| Emotional impact [7, 1] | 3.7 | 3.32 | 3.6 | 3.7 | 0.001 | ||||
| Food anxiety [7, 1] | 3.9 | 3.32 | 3.9 | 3.9 | <0.001 | ||||
| Social and dietary limitations [7, 1] | 3.5 | 2.94 | 3.5 | 3.4 | <0.001 | ||||
| Total QoL [7, 1] | 3.7 | 3.19 | 3.7 | 3.8 | <0.001 | ||||
| Vazquez-Ortiz [50] | pre-OIT M | post-OIT M | Pre-vs. post-OIT | ||||||
| FAQLQ-CF | Children with FA pre-and 12 months post-OIT | Emotional impact [7, 1] | ~4.2 | ~4.0 | 0.218 | ||||
| Allergen avoidance [7, 1] | ~4.3 | ~2.9 | 0.011 | ||||||
| Risk of accidental exposure [7, 1] | ~4.1 | ~3.1 | 0.015 | ||||||
| Dietary restrictions [7, 1] | ~4.5 | ~2.2 | 0.002 | ||||||
| Total QoL [7, 1] | ~4.2 | ~2.9 | 0.014 | ||||||
| FAQLQ-PF | Parents (as proxies) of children with FA pre-and 12 months post-OIT | Emotional impact [7, 1] | ~2.5 | ~2.9 | 0.823 | ||||
| Food anxiety [7, 1] | ~2.8 | ~2.5 | 0.414 | ||||||
| Social and dietary limitations [7, 1] | ~2.4 | ~1.3 | 0.019 | ||||||
| Total QoL [7, 1] | ~2.8 | ~2.3 | 0.164 | ||||||
| de Weger [54] | FAQLQ-CF | Children with FA, recommended to introduce peanut/tree-nut at home | Accepted Introduction m | Declined Introduction m | Accepted vs. declined | ||||
| Emotional impact [7, 1] | 3.00 (1.50–4.33) | 3.67 (2.71–4.88) | 0.367 | ||||||
| Total QoL [7, 1] | 2.70 (1.79–3.96) | 3.98 (3.10–4.26) | 0.161 | ||||||
| FAIM | 2.33 (1.83–3.17) | 2.42 (2.33–3.25) | 0.580 | ||||||
| FAQLQ-PF | Parents (as proxies) of children recommended to introduce peanut/tree-nut at home | Food anxiety [7, 1] | 2.07 (1.38–2.78) | 3.00 (2.44–4.31) | 0.057 | ||||
| Total QoL [7, 1] | 1.92 (1.27–2.45) | 2.75 (2.19–4.17) | 0.062 | ||||||
| FAIM | 2.80 (2.20–3.35) | 3.20 (2.90–4.15) | 0.014 | ||||||
| Study | Instrument | Population | Domain, Score Range [Worst, Best] | QoL Score | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reier-Nilsen [58] | PedsQL 4.0 | Pre-OIT | Post-OIT | Pre-vs. post-OIT | |||||
| Mean (95% CI) | |||||||||
| OIT group-children | Total QoL [0, 100] | 82.1 (79.1–85.2) | 86.7 (83.6–89.7) | <0.0001 | |||||
| OIT group-parents | Total QoL [0, 100] | 79.8 (73.6–83.3) | 88.0 (85.2–90.8) | <0.0001 | |||||
| control children | Total QoL [0, 100] | 83.4 (75.4–91.4) | 82.2 (76.0–88.4) | 0.8 | |||||
| control parents | Total QoL [0, 100] | 81.7 (74.6–88.8) | 82.1 (75.8–88.4) | 0.9 | |||||
| Children | Teens | Pre-vs. post-OFC | |||||||
| van der Valk [53] | Pre-OFC | Post-OFC | Pre-OFC | Post-OFC | |||||
| Mean | Children | Teens | |||||||
| FAQLQ-CF FAQLQ-TF | Children and teens with cashew nut allergy before and 6 months after OFC | Allergen avoidance [7, 1] | 3.06 | 3.57 | 3.45 | 3.24 | 0.102 | 0.392 | |
| Risk of accidental exposure [7, 1] | 3.5 | 3.79 | 3.31 | 3.14 | 0.34 | 0.591 | |||
| Emotional impact, [7, 1] | 3.93 | 3.75 | 3.73 | 3.26 | 0.437 | 0.086 | |||
| Dietary restrictions [7, 1] | 3.44 | 3.43 | NA | NA | 0.97 | NA | |||
| Total QoL [7, 1] | 3.32 | 3.49 | 3.5 | 3.22 | 0.491 | 0.286 | |||
| FAIM | 2.86 | 3.27 | 3.26 | 2.89 | 0.025 | 0.006 | |||
| Children | Teens | OIT vs. placebo | |||||||
| Hourihane [60] | OIT | placebo | OIT | placebo | |||||
| Children and teens who underwent OIT vs. placebo arm | mean change post-OIT | children | teens | ||||||
| FAQLQ-CF FAQLQ-TF | Emotional impact [7, 1] | −0.88 | 0.01 | −0.20 | −0.13 | 0.083 | 0.828 | ||
| Risk of accidental exposure [7, 1] | −0.69 | 0.51 | −0.19 | 0.05 | 0.026 | 0.578 | |||
| Allergen avoidance and dietary restrictions [7, 1] | −0.33 | 0.85 | 0.05 | −0.26 | 0.011 | 0.433 | |||
| Total QoL [7, 1] | −0.64 | 0.45 | −0.19 | −0.05 | 0.015 | 0.640 | |||
| Blumchen [55] | OIT arm | placebo arm | OIT group vs. placebo group | ||||||
| Median (IQR) change | |||||||||
| FAQLQ-CF | Children who underwent OIT vs. placebo arm | Allergen avoidance [7, 1] | −1.9 (−3.0, −0.1) | −0.1 (−0.8, 1.1) | 0.08 | ||||
| Risk of accidental exposure [7, 1] | −2.0 (−3.3, −0.9) | 0.0 (−1.1, 0.8) | 0.02 | ||||||
| Emotional impact, [7, 1] | −1.8 (−2.8, −0.9) | −0.3 (−1.0, 0.9) | 0.02 | ||||||
| Dietary restrictions [7, 1] | −1.2 (−2.8, 0.2) | −0.2 (−1.3, 0.7) | 0.23 | ||||||
| Total QoL [7, 1] | −1.0 (−2.7, −0.5) | −0.1 (−1.2, 0.7) | 0.10 | ||||||
| FAQLQ-PF | Parents (as proxies) of children who underwent OIT vs. placebo arm | Food anxiety [7, 1] | −0.3 (−1.2, 0.8) | −0.1 (−0.7, 0.5) | 0.61 | ||||
| Emotional impact [7, 1] | −0.2 (−1.3, 0.3) | 0.2 (−0.3, 0.5) | 0.17 | ||||||
| Social and dietary limitations [7, 1] | −0.6 (−2.0, 0.1) | −0.1 (−0.6, 0.8) | 0.16 | ||||||
| Total QoL [7, 1] | −0.4 (−1.2, 0.02) | −0.2 (−0.4–0.31) | 0.20 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drakouli, A.-E.; Kontele, I.; Poulimeneas, D.; Saripanagiotou, S.; Grammatikopoulou, M.G.; Sergentanis, T.N.; Vassilakou, T. Food Allergies and Quality of Life among School-Aged Children and Adolescents: A Systematic Review. Children 2023, 10, 433. https://0-doi-org.brum.beds.ac.uk/10.3390/children10030433
Drakouli A-E, Kontele I, Poulimeneas D, Saripanagiotou S, Grammatikopoulou MG, Sergentanis TN, Vassilakou T. Food Allergies and Quality of Life among School-Aged Children and Adolescents: A Systematic Review. Children. 2023; 10(3):433. https://0-doi-org.brum.beds.ac.uk/10.3390/children10030433
Chicago/Turabian StyleDrakouli, Artemis-Eirini, Ioanna Kontele, Dimitrios Poulimeneas, Stella Saripanagiotou, Maria G. Grammatikopoulou, Theodoros N. Sergentanis, and Tonia Vassilakou. 2023. "Food Allergies and Quality of Life among School-Aged Children and Adolescents: A Systematic Review" Children 10, no. 3: 433. https://0-doi-org.brum.beds.ac.uk/10.3390/children10030433