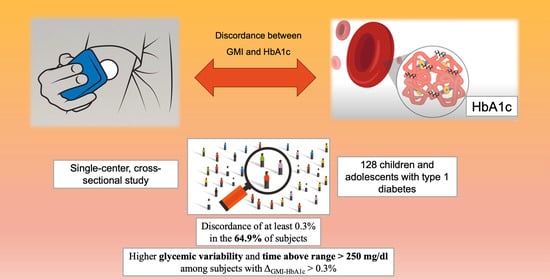
Discordance between Glucose Management Indicator and Glycated Hemoglobin in a Pediatric Cohort with Type 1 Diabetes: A Real-World Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Comparison between Subgroups with Different ΔGMI-HbA1c Values
3.2. Clinical Predictors of ΔGMI-HbA1c
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef]
- Kravarusic, J.; Aleppo, G. Diabetes Technology Use in Adults with Type 1 and Type 2 Diabetes. Endocrinol. Metab. Clin. N. Am. 2020, 49, 37–55. [Google Scholar] [CrossRef]
- Ferrito, L.; Passanisi, S.; Bonfanti, R.; Cherubini, V.; Minuto, N.; Schiaffini, R.; Scaramuzza, A. Efficacy of advanced hybrid closed loop systems for the management of type 1 diabetes in children. Minerva Pediatr. 2021, 73, 474–485. [Google Scholar] [CrossRef]
- Dos Santos, T.J.; Campos, J.d.M.D.; Argente, J.; Rodríguez-Artalejo, F. Effectiveness and equity of continuous subcutaneous insulin infusions in pediatric type 1 diabetes: A systematic review and meta-analysis of the literature. Diabetes Res. Clin. Pract. 2021, 172, 108643. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Nimri, R.; Dovc, K.; Phillip, M.; Bratina, N. Prevention of Hypoglycemia with Predictive Low Glucose Insulin Suspension in Children with Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care 2017, 40, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Rusak, E.; Ogarek, N.; Wolicka, K.; Mrówka, A.; Seget, S.; Kuźnik, M.; Jarosz-Chobot, P. The Quality of Life and Satisfaction with Con-tinuous Glucose Monitoring Therapy in Children under 7 Years of Age with T1D Using the rtCGM System Integrated with Insulin Pump-A Caregivers Point of View. Sensors 2021, 21, 3683. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; So, W.K.; Leung, D.Y. Effectiveness of Continuous Subcutaneous Insulin Infusion on Parental Quality of Life and Glycemic Control Among Children with T1D: Meta-Analysis. Worldviews Evid.-Based Nurs. 2018, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Vehik, K.; Cuthbertson, D.; Boulware, D.; Beam, C.A.; Rodriguez, H.; Legault, L.; Hyytinen, M.; Rewers, M.J.; Schatz, D.A.; Krischer, J.P. Performance of HbA1c as an early diagnostic indicator of type 1 diabetes in children and youth. Diabetes Care 2012, 35, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Lenters-Westra, E.; Schindhelm, R.K.; Bilo, H.J.; Slingerland, R.J. Haemoglobin A1c: Historical overview and current concepts. Diabetes Res. Clin. Pract. 2013, 99, 75–84. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Codner, E.; Craig, M.E.; Huynh, T.; Maahs, D.M.; Mahmud, F.H.; Marcovecchio, L.; DiMeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr. Diabetes 2022, 23, 1270–1276. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef]
- Andreasson, R.; Ekelund, C.; Landin-Olsson, M.; Nilsson, C. HbA1c levels in children with type 1 diabetes and correlation to diabetic retinopathy. J. Pediatr. Endocrinol. Metab. 2018, 31, 369–374. [Google Scholar] [CrossRef]
- Lind, M.; Pivodic, A.; Svensson, A.M.; Ólafsdóttir, A.F.; Wedel, H.; Ludvigsson, J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019, 366, l4894. [Google Scholar] [CrossRef]
- Samuelsson, U.; Steineck, I.; Gubbjornsdottir, S. A high mean-HbA1c value 3–15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood—A pilot study using two nation-wide population based quality registries. Pediatr. Diabetes 2014, 15, 229–235. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; et al. 7. Diabetes Technology: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S111–S127. [Google Scholar] [CrossRef]
- Friedman, J.G.; Cardona Matos, Z.; Szmuilowicz, E.D.; Aleppo, G. Use of Continuous Glucose Monitors to Manage Type 1 Diabetes Mellitus: Progress, Challenges, and Recommendations. Pharmacogenom. Pers. Med. 2023, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous glucose monitoring systems—Current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L.M.; Kanapka, L.G.; Beck, R.W.; Bergamo, K.; Clements, M.A.; Criego, A.; DeSalvo, D.J.; Goland, R.; Hood, K.; Liljenquist, D.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults with Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2020, 323, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019, 7, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Beck, R.W.; Close, K.L.; Grunberger, G.; Sacks, D.B.; Kowalski, A.; Brown, A.S.; Heinemann, L.; Aleppo, G.; Ryan, D.B.; et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C from Continuous Glucose Monitoring. Diabetes Care 2018, 41, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Piona, C.; Marigliano, M.; Mozzillo, E.; Di Candia, F.; Zanfardino, A.; Iafusco, D.; Maltoni, G.; Zucchini, S.; Piccinno, E.; Maffeis, C. Evaluation of HbA1c and glucose management indicator discordance in a population of children and adolescents with type 1 diabetes. Pediatr. Diabetes 2022, 23, 84–89. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Mortensen, H.B.; Hougaard, P.; Swift, P.; Hansen, L.; Holl, R.W.; Hoey, H.; Bjoerndalen, H.; de Beaufort, C.; Chiarelli, F.; Danne, T.; et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009, 32, 1384–1390. [Google Scholar] [CrossRef]
- Battelino, T.; The SWITCH Study Group; Conget, I.; Olsen, B.; Schütz-Fuhrmann, I.; Hommel, E.; Hoogma, R.; Schierloh, U.; Sulli, N.; Bolinder, J. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: A randomised controlled trial. Diabetologia 2012, 55, 3155–3162. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults with Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Lind, M.; Polonsky, W.; Hirsch, I.B.; Heise, T.; Bolinder, J.; Dahlqvist, S.; Schwarz, E.; Ólafsdóttir, A.F.; Frid, A.; Wedel, H.; et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults with Type 1 Diabetes Treated with Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA 2017, 317, 379–387. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insu-lin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Cox, D.J.; Kovatchev, B.P.; Julian, D.M.; Gonder-Frederick, L.A.; Polonsky, W.H.; Schlundt, D.G.; Clarke, W.L. Frequency of severe hy-poglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J. Clin. Endocrinol. Metab. 1994, 79, 1659–1662. [Google Scholar]
- Monzon, A.D.; Patton, S.R.; Clements, M. An Examination of the Glucose Management Indicator in Young Children with Type 1 Diabetes. J. Diabetes Sci. Technol. 2022, 16, 1505–1512. [Google Scholar] [CrossRef]
- Lombardo, F.; Salzano, G.; Bombaci, B.; Basile, P.; Lucania, G.; Alibrandi, A.; Passanisi, S. Has COVID-19 lockdown improved gly-caemic control in pediatric patients with type 1 diabetes? An analysis of continuous glucose monitoring metrics. Diabetes Res. Clin. Pract. 2021, 178, 108988. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.E.; Gooley, T.A.; McNulty, B.; Meyers, J.; Hirsch, I.B. HbA1c and Glucose Management Indicator Discordance: A Real-World Analysis. Diabetes Technol. Ther. 2021, 23, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Lu, J.; Zhou, J. Glycemic variability: Measurement, target, impact on complications of diabetes and does it really matter? J. Diabetes Investig. 2023, 15, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Piona, C.; Ventrici, C.; Marcovecchio, L.; Chiarelli, F.; Maffeis, C.; Bonfanti, R.; Rabbone, I. Long-term complications of type 1 diabetes: What do we know and what do we need to understand? Minerva Pediatr. 2021, 73, 504–522. [Google Scholar] [CrossRef]
- Lombardo, F.; Passanisi, S.; Alibrandi, A.; Bombaci, B.; Bonfanti, R.; Delvecchio, M.; Di Candia, F.; Mozzillo, E.; Piccinno, E.; Piona, C.A.; et al. MiniMed 780G Six-Month Use in Children and Adolescents with Type 1 Diabetes: Clinical Targets and Predictors of Optimal Glucose Control. Diabetes Technol. Ther. 2023, 25, 404–413. [Google Scholar] [CrossRef]
- Arrieta, A.; Battelino, T.; Scaramuzza, A.E.; Da Silva, J.; Castañeda, J.; Cordero, T.L.; Shin, J.; Cohen, O. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidence from 12870 real-world users. Diabetes Obes. Metab. 2022, 24, 1370–1379. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Passanisi, S.; Piona, C.; Salzano, G.; Marigliano, M.; Bombaci, B.; Morandi, A.; Alibrandi, A.; Maffeis, C.; Lombardo, F. Aiming for the Best Glycemic Control Beyond Time in Range: Time in Tight Range as a new CGM Metric in Children and Adolescents with Type 1 Diabetes using Different Treatment Modalities. Diabetes Technol. Ther. 2023; ahead of print. [Google Scholar] [CrossRef]
- Rebesco, D.B.; França, S.N.; de Lima, V.A.; Leite, N.; Smouter, L.; de Souza, W.C.; Komatsu, W.R.; Mascarenhas, L.P.G. Different amounts of moderate to vigorous physical activity and change in glycemic variability in adolescents with type 1 diabetes: Is there dose-response relationship? Arch. Endocrinol. Metab. 2020, 64, 312–318. [Google Scholar] [CrossRef]
- Zhu, J.; Volkening, L.K.; Laffel, L.M. Distinct Patterns of Daily Glucose Variability by Pubertal Status in Youth with Type 1 Diabetes. Diabetes Care 2020, 43, 22–28. [Google Scholar] [CrossRef]
- Monzon, A.D.; Smith, L.B.; Powers, S.W.; Dolan, L.M.; Patton, S.R. The Association Between Glycemic Variability and Mac-ronutrients in Young Children with T1D. J. Pediatr. Psychol. 2020, 45, 749–758. [Google Scholar] [CrossRef]
- İpar, N.; Boran, P.; Barış, H.E.; Us, M.C.; Aygün, B.; Haliloğlu, B.; Gökçe, T.; Can, E.; Eviz, E.; İnan, N.G.; et al. Corrigendum to “Associations between sleep characteristics and glycemic variability in youth with type 1 diabetes”. Sleep Med. 2023, 109, 132–142, Erratum in Sleep Med. 2023, 113, 422. [Google Scholar] [CrossRef]
- Lombardo, F.; Bombaci, B.; Alibrandi, A.; Visalli, G.; Salzano, G.; Passanisi, S. The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes. Children 2022, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, A. Glycaemic control and haemoglobinopathy: When HbA1C may not be reliable. Diabetes Spectr. 2008, 21, 46–49. [Google Scholar] [CrossRef]
- Mozzillo, E.; Franceschi, R.; Piona, C.; Passanisi, S.; Casertano, A.; Pjetraj, D.; Maltoni, G.; Calcaterra, V.; Cauvin, V.; Cherubini, V.; et al. Diabetes and Prediabetes in Children with Cystic Fibrosis: A Systematic Review of the Literature and Recommendations of the Italian Society for Pediatric Endocrinology and Diabetes (ISPED). Front. Endocrinol. 2021, 12, 673539. [Google Scholar] [CrossRef]
- Zirilli, G.; Salzano, G.; Galletta, F.; Basile, P.; Caime, F.; Cucinotta, U.; Licciardello, M.; Passanisi, S.; Russo, G.; Lombardo, F. Vitamin B12 deficiency in type 1 diabetes patient with thalassemia trait: The challenge of early diagnosis. J. Biol. Regul. Homeost. Agents 2022, 36, 123–127. [Google Scholar]
- Cohen, R.M.; Franco, R.S.; Khera, P.K.; Smith, E.P.; Lindsell, C.J.; Ciraolo, P.J.; Palascak, M.B.; Joiner, C.H. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008, 112, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
| Variables | Frequency and Mean ± SDS |
|---|---|
| Number of subjects | 128 |
| Age (years) | 13.4 ± 3.6 |
| Duration of diabetes (ys) | 5.8 ± 9 |
| Age at onset (ys) | 7.6 ± 3.8 |
| Ethnicity Caucasian Others | 127 (99.2%) 1 (0.8%) |
| Biological sex Male Female | 73 (57%) 55 (43%) |
| Comorbidities Yes No | 27 (21.1%) 101 (78.9%) |
| BMI Z-score | 0.5 ± 0.91 |
| HbA1C (%) | 6.7 ± 0.7 |
| GMI (%) | 7.0 ± 0.6 |
| Sensor use (%) | 92.3 ± 9.5 |
| Glucose monitoring system isCGM rtCGM | 20 (15.6%) 108 (84.4%) |
| Insulin treatment type MDI SAP PLGS HCL AHCL | 27 (21.1%) 19 (14.8%) 11 (8.6%) 8 (6.3%) 63 (49.2%) |
| Δ ≤ −0.3% (n = 29) | −0.3% < Δ ≤ 0.3% (n = 45) | Δ > 0.3% (n = 54) | p | Cohort | |
|---|---|---|---|---|---|
| Age (years) | 12.8 ± 3.2 | 13.2 ± 3.4 | 13.7 ± 3.8 | 0.724 | 13.4 ± 3.6 |
| BMI (Z-score) | 0.66 ± 1 | 0.56 ± 0.99 | 0.5 ± 0.83 | 0.532 | 0.5 ± 0.91 |
| Mean sensor glucose (mg/dL) | 172.7 ± 20.6 | 150.8 ± 13.4 | 134.7 ± 17.8 | <0.001 * | 147.9 ± 25 |
| CV (%) | 35.2 ± 4.2 | 34.5 ± 4.4 | 38 ± 6.2 | <0.001 * | 36.2 ± 5.4 |
| TIR (%) | 71.1 ± 11.1 | 70.6 ± 10.5 | 67.8 ± 14.1 | 0.407 | 69.2 ± 12.4 |
| TARLevel1 (%) | 19.7 ± 6.2 | 20.9 ± 7.3 | 19 ± 7.2 | 0.421 | 20.1 ± 7 |
| TARLevel2 (%) | 6.1 ± 5.8 | 5.5 ± 4.6 | 9 ± 8.9 | 0.033 * | 7.3 ± 7.1 |
| TBRLevel1 (%) | 2.5 ± 1.8 | 2.4 ± 2.4 | 3 ± 2.2 | 0.276 | 2.7 ± 2.2 |
| TBRLevel2 (%) | 0.5 ± 0.9 | 0.5 ± 0.8 | 0.8 ± 0.8 | 0.24 | 0.6 ± 0.8 |
| RBC (million/mm3) | 5.04 ± 4.63 | 4.97 ± 3.41 | 5.10 ± 5.53 | 0.391 | 5.03 ± 4.69 |
| Hb (g/dL) | 14 ± 1.3 | 14.1 ± 1.1 | 14.4 ± 1.5 | 0.294 | 14.2 ± 1.3 |
| Hct (%) | 40.8 ± 3.2 | 41.4 ± 3.1 | 42 ± 4 | 0.593 | 41.5 ± 3.5 |
| MCV (fL) | 81.6 ± 5.4 | 83.6 ± 4.6 | 83.3 ± 7.6 | 0.388 | 83.1 ± 6.2 |
| MCH (pg) | 28 ± 2.3 | 28.5 ± 1.8 | 28.4 ± 2.7 | 0.656 | 28.4 ± 2.3 |
| MCHC (%) | 34.1 ± 1.4 | 33.9 ± 0.7 | 33.9 ± 1 | 0.674 | 33.9 ± 1 |
| RDW-CV (%) | 13.4 ± 0.7 | 13.3 ± 0.7 | 13.3 ± 0.9 | 0.711 | 13.3 ± 0.8 |
| WBC (n/mm3) | 6652.7 ± 1518 | 6614.9 ± 1725.8 | 6784 ± 1861.3 | 0.881 | 6694.8 ± 1729.7 |
| PLT (n/mm3) | 285,379.3 ± 59,613 | 271,555 ± 53,927 | 283,962 ± 67,026 | 0.520 | 279,921 ± 60,864 |
| Univariate Linear Regression | |||
| Variable | B | 95% CI | p-Value |
| Biological sex (male) | −0.029 | −0.231; 0.174 | 0.781 |
| Age | 0.014 | −0.014; 0.042 | 0.310 |
| Duration of diabetes | 0.005 | −0.022; 0.032 | 0.699 |
| Comorbidities | −0.001 | −0.247; 0.245 | 0.995 |
| BMI Z score | −0.052 | −0.160; 0.056 | 0.340 |
| Mean sensor glucose | −0.014 | −0.017; −0.011 | <0.001 * |
| RBC | 0.001 | −0.001; 0.003 | 0.456 |
| Hemoglobin | 0.043 | −0.032; 0.118 | 0.258 |
| Hct | 0.021 | −0.007; 0.049 | 0.131 |
| MCV | 0.008 | −0.008; 0.024 | 0.302 |
| MCH | 0.013 | −0.030; 0.056 | 0.546 |
| MCHC | −0.043 | −0.141; 0.054 | 0.380 |
| RDW-CV | −0.054 | −0.179; 0.071 | 0.395 |
| WBC | 0.000 | −0.006; 0.005 | 0.867 |
| Sensor use | 0.007 | −0.005; 0.018 | 0.250 |
| CV | 0.014 | −0.005; 0.032 | 0.140 |
| TIR | −0.004 | −0.013; 0.004 | 0.276 |
| TARLevel1 | −0.001 | −0.015; 0.013 | 0.908 |
| TARLevel2 | 0.012 | 0.002; 0.026 | 0.045 * |
| TBRLevel1 | 0.009 | −0.036; 0.054 | 0.697 |
| TBRLevel2 | 0.045 | −0.072; 0.162 | 0.445 |
| Multivariate Linear Regression | |||
| Variable | B | 95% CI | p-Value |
| Biological sex (male) | −0.074 | −0.206; 0.057 | 0.265 |
| Age | 0.010 | −0.010; 0.030 | 0.329 |
| Duration of diabetes | 0.007 | −0.010; 0.024 | 0.402 |
| Comorbidities | −0.40 | −0.184; 0.104 | 0.584 |
| BMI Z score | −0.034 | −0.091; 0.022 | 0.226 |
| Mean sensor glucose | −0.023 | −0.026; −0.021 | <0.001 * |
| RBC | 0.008 | −0.001; 0.017 | 0.071 |
| Hb | −0.210 | −0.814; 0.394 | 0.492 |
| Hct | −0.023 | −0.197; 0.150 | 0.790 |
| MCV | 0.149 | −0.011; 0.310 | 0.067 |
| MCH | −0.291 | −0.760; 0.178 | 0.222 |
| MCHC | 0.313 | −0.169; 0.795 | 0.201 |
| RDW-CV | −0.037 | −0.130; 0.057 | 0.438 |
| WBC | −0.001 | −0.004; 0.002 | 0.528 |
| Sensor use | 0.003 | −0.004; 0.010 | 0.376 |
| CV | 0.016 | −0.004; 0.035 | 0.114 |
| TIR | 0.018 | −0.042; 0.079 | 0.550 |
| TARLevel1 | 0.039 | −0.022; 0.100 | 0.206 |
| TARLevel2 | 0.053 | 0.002; 0.105 | 0.043 * |
| TBRLevel1 | −0.019 | −0.085; 0.048 | 0.578 |
| TBRLevel2 | −0.001 | −0.089; 0.087 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti Randazzese, S.; Bombaci, B.; Costantino, S.; Giorgianni, Y.; Lombardo, F.; Salzano, G. Discordance between Glucose Management Indicator and Glycated Hemoglobin in a Pediatric Cohort with Type 1 Diabetes: A Real-World Study. Children 2024, 11, 210. https://0-doi-org.brum.beds.ac.uk/10.3390/children11020210
Foti Randazzese S, Bombaci B, Costantino S, Giorgianni Y, Lombardo F, Salzano G. Discordance between Glucose Management Indicator and Glycated Hemoglobin in a Pediatric Cohort with Type 1 Diabetes: A Real-World Study. Children. 2024; 11(2):210. https://0-doi-org.brum.beds.ac.uk/10.3390/children11020210
Chicago/Turabian StyleFoti Randazzese, Simone, Bruno Bombaci, Serena Costantino, Ylenia Giorgianni, Fortunato Lombardo, and Giuseppina Salzano. 2024. "Discordance between Glucose Management Indicator and Glycated Hemoglobin in a Pediatric Cohort with Type 1 Diabetes: A Real-World Study" Children 11, no. 2: 210. https://0-doi-org.brum.beds.ac.uk/10.3390/children11020210