Effectiveness and Coverage of Treatment for Severe Acute Malnutrition Delivered by Community Health Workers in the Guidimakha Region, Mauritania
Abstract
:1. Introduction
2. Materials and Methods
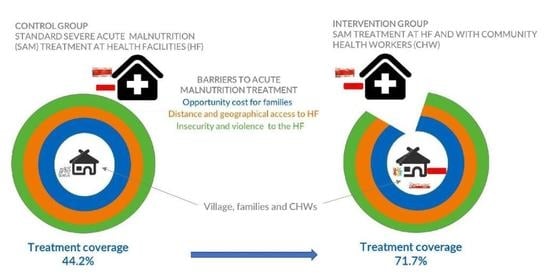
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OCHA. Sahel Overview of Humanitarian Crises and Needs. 2018. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/sahel_hnro_2018_0.pdf (accessed on 5 May 2020).
- UNICEF. Mauritania Humanitarian Situation Report. 2019. Available online: https://www.unicef.org/media/79541/file/Mauritania-SitRep-September-2019.pdf (accessed on 5 May 2020).
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers 2017, 3, 17067. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
- Pelletier, D.L.; Frongillo EAJr Schroeder, D.G.; Habicht, J.P. The effects of malnutrition on child mortality in developing countries. Bull. World Health Organ. 1995, 73, 443–448. [Google Scholar] [PubMed]
- Chevalier, P.; Delpeuch, F.; Maire, B. Le complexe “malnutrition-infection”: Premier problème de santé publique chez les populations défavorisées. Méd. Mal. Infect. 1996, 26 (Suppl. 3), 366–370. [Google Scholar] [CrossRef]
- de Onis, M.; Borghi, E.; Arimond, M.; Webb, P.; Croft, T.; Saha, K.; De-Regil, L.M.; Thuita, F.; Heidkamp, R.; Krasevec, J.; et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2019, 22, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, E.; Myatt, M.; Woodhead, S.; Guerrero, S.; Alvarez, J.L. Coverage of community-based management of severe acute malnutrition programs in twenty-one countries, 2012–2013. PLoS ONE 2015, 10, e0128666. [Google Scholar] [CrossRef] [Green Version]
- Plan National de Développement Sanitaire 2017–2020. Volume 1: Analyse de Situation, Ministère de la Santé Mauritanie, Février 2017. Available online: https://www.sante.gov.mr/?wpfb_dl=203 (accessed on 5 May 2020).
- Link NCA. Nutrition Causal Analyses. Mauritanie, Région de Guidimakha. 2017. Available online: https://www.linknca.org/etude/region_de_guidimakha.htm (accessed on 6 May 2020).
- UNICEF. La stratégie National Communautaire de Santé en Mauritanie. Juin 2012. Available online: http://eps-ms.mr/la-strategie-nationale-communautaire-de-sante-en-mauritanie/ (accessed on 1 December 2021).
- UNICEF, GLOBAL NUTRITION CLUSTER, GTAM 2019. Nutrition Information Management, Surveillance and Monitoring in the Context of COVID-19. Available online: https://www.nutritioncluster.net/sites/nutritioncluster.com/files/2020-07/Nutrition-information-COVID-Brief%232%20Final%20July2020.pdf (accessed on 6 May 2020).
- United Nations Children’s Fund (UNICEF). Integrating Early Detection and Treatment of Child; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2021. [Google Scholar]
- Wasting into Routine Primary Health Care Services: A Resource Guide to Support National Planning. UNICEF, UNICEF, New York. Available online: https://www.unicef.org/documents/integrating-wasting-treatment (accessed on 5 November 2021).
- City Population. Mauritania. Administrative Division. Available online: https://citypopulation.de/en/mauritania/admin/101__ould_yeng%C3%A9/ (accessed on 10 October 2021).
- Enquête Nutritionnelle Nationale Utilisant la Méthodologie SMART Mauritanie. 2017. Available online: https://reliefweb.int/map/mauritania/mauritanie-zones-de-laic-et-pr-valence-de-la-malnutrition-aigu-globale-6-ao-t-2017 (accessed on 1 December 2021).
- ENA for SMART—Software for Emergency Nutrition Assessment. Available online: http://www.nutrisurvey.de/ena/ena.html (accessed on 6 May 2020).
- Ministère de la Santé, République Islamique de Mauritanie. Protocole National de Prise en Charge de la Malnutrition Aigüe. 2011. Available online: https://www.sante.gov.mr/?page_id=310 (accessed on 1 December 2021).
- Zangenberg, M.; Abdissa, A.; Johansen, Ø.H.; Tesfaw, G.; Friis, H.; Briend, A.; Beza, E.; Jørgen, A.L.K.; Tsinuel, G. Critical evaluation of the appetite test for children with severe acute malnutrition. Trop. Med. Int. Health 2020, 25, 424–432. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. The WHO Anthro Software. Available online: https://www.who.int/childgrowth/software/en/ (accessed on 20 January 2021).
- Myatt, M.; Guevarra, E.; Fieschi, L.; Norris, A.; Guerrero, S.; Schofield, L.; Jones, D.; Emru, E.; Sadler, K. Semi-Quantitative Evaluation of Access and Coverage (SQUEAC)/Simplified Lot Quality Assurance Sampling Evaluation of Access and Coverage (SLEAC) Technical Reference. 2012. Available online: https://www.fantaproject.org/sites/default/files/resources/SQUEAC-SLEAC-Technical-Reference-Oct2012_0.pdf (accessed on 20 January 2021).
- The Sphere Project. Minimum Standards in Food Security and Nutrition. Humanitarian Charter and Minimum Standards in Humanitarian Response. Sphere Handbook. 2018. Available online: https://spherestandards.org/es/el-manual/ (accessed on 20 May 2021).
- Wilunda, C.; Mumba, F.G.; Putoto, G.; Maya, G.; Musa, E.; Lorusso, V.; Magige, C.; Leyna, G.; Manenti, F.; Dalla Riva, D.; et al. Effectiveness of screening and treatment of children with severe acute malnutrition by community health workers in Simiyu region, Tanzania: A quasi-experimental pilot study. Sci. Rep. 2021, 11, 2342. [Google Scholar] [CrossRef] [PubMed]
- Kozuki, N.; Van Boetzelaer, E.; Tesfai, C.; Zhou, A. Severe acute malnutrition treatment delivered by low-literate community health workers in South Sudan: A prospective cohort study. J. Glob. Health 2020, 10, 010421. [Google Scholar] [CrossRef] [PubMed]
- López-Ejeda, N.; Charle-Cuéllar, P.; Vargas, A.; Guerrero, S. Can community health workers manage uncomplicated severe acute malnutrition? A review of operational experiences in delivering severe acute malnutrition treatment through community health platforms. Matern. Child Nutr. 2019, 15, e12719. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.; Puett, C.; Mothabbir, G.; Myatt, M.; Community Case Management of Severe Acute Malnutrition in Southern Bangladesh. Save the Children, Feinstein International Center, Tufts University. 2011. Available online: https://fic.tufts.edu/assets/Community-Case-Mgt.pdf (accessed on 2 April 2021).
- Morgan, S.; Bulten, R.; Jalipa, D.H. Community Case Management Approach to SAM Treatment in Angola. Field Exchange 49. March 2015, p. 3. Available online: https://www.ennonline.net/fex/49/angola (accessed on 17 May 2021).
- Alvarez Morán, J.L.; Alé, G.B.F.; Charle, P.; Sessions, N.; Doumbia, S.; Guerrero, S. The effectiveness of treatment for Severe Acute Malnutrition (SAM) delivered by community health workers compared to a traditional facility based model. BMC Health Serv. Res. 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulu, J.M.; Perry, H.B. Community health workers at the dawn of a new era. Health Res. Policy Syst. 2021, 19 (Suppl. 3), 130. [Google Scholar] [CrossRef] [PubMed]
- López-Ejeda, N.; Charle-Cuellar, P.; GB Alé, F.; Álvarez, J.L.; Vargas, A.; Guerrero, S. Bringing severe acute malnutrition treatment close to households through community health workers can lead to early admissions and improved discharge outcomes. PLoS ONE 2020, 15, e0227939. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Habicht, J.P.; Bryce, J. Evidence-based public health: Moving beyond randomized trials. Am. J. Public Health 2004, 94, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C. Learning and knowledge-production for public health: A review of approaches to evidence-based public health. Scand J. Public Health 2000, 28, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Sadler, K.; Dent, N.; Khara, T.; Guerrero, S.; Myatt, M.; Saboya, M.; Walsh, A. Key issues in the success of community-based management of severe malnutrition. Food Nutr. Bull. 2006, 27 (Suppl. 3), S49–S82. [Google Scholar] [CrossRef] [PubMed]
- WHO, WFP, UNICEF. Global Action Plan on Child Wasting: A Framework for Action to Accelerate Progress in Preventing and Managing Child Wasting and the Achievement of the Sustainable Development Goals. [Internet]. Available online: https://www.who.int/publications/m/item/global-action-plan-on-child-wasting-a-framework-for-action (accessed on 13 September 2021).
- UNICEF. Simplified Approaches for the Treatment of Wasting. Available online: https://www.simplifiedapproaches.org/ (accessed on 26 May 2021).

| Control | Intervention | p Value | |||
|---|---|---|---|---|---|
| n | % (95% C.I.) | n | % (95% C.I.) | ||
| Demographics | 724 | 730 | |||
| Female proportion | 310 | 42.8 (40.2–45.5) | 332 | 45.5 (42.8–48.1) | 0.307 |
| 6–59 month children | 501 | 69.2 (65.7–72.6) | 518 | 71.0 (67.7–74.3) | 0.464 |
| Global Acute Malnutrition | 36 | 7.2 (5.2–9.8) | 34 | 6.6 (4.7–9.0) | 0.695 |
| Severe Acute Malnutrition | 2 | 0.4 (0.1–1.4) | 2 | 0.4 (0.4–1.4) | 0.973 |
| House characteristics | 223 | 212 | |||
| Cement floor | 17 | 7.6 (4.5–11.9) | 52 | 24.5 (18.9–30.9) | <0.001 |
| Handmade earth brick roof | 50 | 22.4 (17.1–28.5) | 36 | 17.0 (12.2–22.7) | 0.154 |
| House in property | 197 | 88.3 (83.4–92.2) | 179 | 84.4 (78.8–89.0) | 0.234 |
| Potable water in the house | 12 | 5.4 (2.8–9.2) | 18 | 8.5 (5.1–13.1) | 0.201 |
| Potable water closes to household | 111 | 49.8 (43.0–56.5) | 92 | 43.4 (36.6–50.4) | 0.185 |
| Health care access barriers | 223 | 212 | |||
| Cost | 112 | 50.2 (0.43–0.57) | 101 | 47.6 (40.8–54.6) | 0.590 |
| Distance | 107 | 48.0 (41.3–54.8) | 105 | 49.5 (42.6–56.5) | 0.747 |
| Family disagrees | 4 | 1.8 (0.5–4.5) | 6 | 2.9 (1.1–6.1) | |
| Sick child treatment preference | 223 | 212 | |||
| Medication of health center | 124 | 55.6 (48.8–62.2) | 170 | 80.2 (74.2–85.3) | <0.001 |
| Traditional self-medication (herbs) | 15 | 6.8 (3.8–10.9) | 9 | 4.2 (2.0–7.9) | 0.257 |
| Self-medication (street drugs) | 28 | 12.5 (8.5–17.6) | 15 | 7.1 (4.0–11.4) | 0.556 |
| Traditional medicine | 56 | 25.1 (19.6–31.3) | 18 | 8.5 (5.1–13.1) | <0.001 |
| Study Groups | Control | Intervention | p Value |
|---|---|---|---|
| MUAC indicators | n = 251 median (IQR) | n = 618 median (IQR) | |
| MUAC (mm) | 112 (115–120) | 112 (115–120) | 0.478 |
| MUAC quartiles * | % (n) | % (n) | |
| Q1 < 110 mm | 8.4 (21) | 9.1 (56) | 0.744 |
| Q2 ≥ 110 mm to <115 mm | 32.3 (81) | 31.7 (196) | 0.873 |
| Q3 ≥ 115 mm to <120 mm | 24.7 (62) | 26.4 (163) | 0.610 |
| Q4 ≥ 120 mm | 34.7 (87) | 32.8 (203) | 0.030 |
| Weight-for-Height indicators | n = 239 median (IQR) | n = 601 median (IQR) | p value |
| Weight (kg) | 6.70 (5.90–7.50) | 6.70 (5.95–7.50) | 0.854 |
| Height (cm) | 72.0 (67.0–77.0) | 72.0 (67.5–77.0) | 0.374 |
| WHZ | −3.26 (−3.61–−2.80) | −3.31 (−3.84–−2.78) | 0.135 |
| WHZ ranges * | % (n) | % (n) | |
| Q1 < −3.76 | 20.9 (50) | 27.3 (164) | 0.056 |
| Q2 ≥ −3.76 to <−3.29 | 25.5 (61) | 24.5 (147) | 0.747 |
| Q3 ≥ −3.29 to <−2.78 | 29.3 (70) | 24.1 (145) | 0.122 |
| Q4 ≥ −2.78 | 24.3 (58) | 24.1 (145) | 0.966 |
| Intervention Group | Health Staff | CHWs | p Value |
|---|---|---|---|
| MUAC indicators | n = 512 median (IQR) | n = 106 median (IQR) | |
| MUAC (mm) | 115 (111–120) | 116 (114–120) | 0.015 |
| MUAC quartiles * | % (n) | % (n) | |
| Q1 < 110 mm | 10.6 (54) | 1.9 (2) | 0.005 |
| Q2 ≥ 110 mm to <115 mm | 32.0 (164) | 30.2 (32) | 0.711 |
| Q3 ≥ 115 mm to <120 mm | 25.4 (130) | 31.1 (33) | 0.222 |
| Q4 ≥ 120 mm | 32.0 (164) | 36.8 (39) | 0.623 |
| Weight-for-Height indicators | n = 496 median (IQR) | n = 105 median (IQR) | p value |
| Weight (kg) | 6.70 (5.90–7.48) | 6.80 (6.15–7.80) | 0.179 |
| Height (cm) | 72.0 (67.0–77.0) | 75.2 (98.7–78.0) | 0.370 |
| WHZ | −3.31 (−3.86–−2.79) | −3.31 (−3.75–−2.63) | 0.366 |
| WHZ quartiles * | % (n) | % (n) | |
| Q1 < −3.76 | 27.8 (138) | 24.8 (26) | 0.522 |
| Q2 ≥ −3.76 to <−3.29 | 24.0 (119) | 26.7 (28) | 0.565 |
| Q3 ≥ −3.29 to <−2.78 | 25.4 (126) | 18.1 (19) | 0.112 |
| Q4 ≥ −2.78 | 22.8 (113) | 30.5 (32) | 0.094 |
| Whole Sample | Control (n = 209) | Intervention (n = 496) | Comparison | ||
|---|---|---|---|---|---|
| Treatment outcomes | n | % | n | % | HR (95% C.I.); p value 2 |
| Cured | 172 | 82.3 | 379 | 76.4 | 0.967 (0.807–1.159); 0.719 |
| Default | 8 | 3.8 | 18 | 3.6 | 0.915 (0.395–2.121); 0.836 |
| Nonrespondent | 0 | 0 | 0 | 0 | |
| Medical reference | 20 | 9.6 | 67 | 13.5 | 1.297 (0.733–2.294); 0.732 |
| Internal transference | 9 | 4.3 | 32 | 6.5 | 1.659 (0.760–3.625); 0.204 |
| Death | 0 | 0 | 0 | 0 | |
| Anthropometric gain 1 | n | Median (IQR) | n | Median (IQR) | p value 3 |
| Total weight (g/kg) | 161 | 197.2 (157.9–254.3) | 356 | 209.7 (164.6–255.2) | 0.283 |
| Daily weight (g/kg/day) | 161 | 4.68 (3.17–7.11) | 356 | 4.73 (3.39–7.35) | 0.426 |
| Total MUAC (mm) | 168 | 11.0 (8.0–15.0) | 364 | 13.0 (9.0–16.0) | 0.059 |
| Daily MUAC (mm/day) | 168 | 0.27 (0.17–0.41) | 363 | 0.29 (0.20–0.43) | 0.139 |
| Intervention Group | HEALTH STAFF (n = 418) | CHWs (n = 78) | Comparison | ||
|---|---|---|---|---|---|
| Treatment outcomes | n | % | n | % | HR (95% C.I.); p value 2 |
| Cured | 319 | 76.3 | 60 | 76.9 | 1.135 (0.860–1.498); 0.373 |
| Default | 16 | 3.8 | 2 | 2.6 | 0.384 (0.051–2.909); 0.354 |
| Nonrespondent | 0 | 0 | 0 | 0 | |
| Medical reference | 64 | 15.3 | 3 | 3.8 | 0.246 (0.060–1.019); 0.053 |
| Internal transference | 19 | 4.5 | 13 | 16.7 | 4.436 (2.146–9.170); <0.001 |
| Death | 0 | 0 | 0 | 0 | |
| Anthropometric gain 1 | n | Median (IQR) | n | Median (IQR) | p value 3 |
| Total weight (g/kg) | 298 | 211.4 (163.9–261.5) | 58 | 196.2 (168.4–232.5) | 0.403 |
| Daily weight (g/kg/day) | 298 | 4.63 (3.35–7.54) | 58 | 5.49 (3.76–8.38) | 0.056 |
| Total MUAC (mm) | 305 | 13.0 (9.5–17.0) | 59 | 12.0 (7.0–14.0) | 0.011 |
| Daily MUAC (mm/day) | 305 | 0.29 (0.19–0.43) | 59 | 0.29 (0.21–0.48) | 0.749 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charle-Cuéllar, P.; Lopez-Ejeda, N.; Toukou Souleymane, H.; Yacouba, D.; Diagana, M.; Dougnon, A.O.; Vargas, A.; Briend, A. Effectiveness and Coverage of Treatment for Severe Acute Malnutrition Delivered by Community Health Workers in the Guidimakha Region, Mauritania. Children 2021, 8, 1132. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121132
Charle-Cuéllar P, Lopez-Ejeda N, Toukou Souleymane H, Yacouba D, Diagana M, Dougnon AO, Vargas A, Briend A. Effectiveness and Coverage of Treatment for Severe Acute Malnutrition Delivered by Community Health Workers in the Guidimakha Region, Mauritania. Children. 2021; 8(12):1132. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121132
Chicago/Turabian StyleCharle-Cuéllar, Pilar, Noemí Lopez-Ejeda, Hassane Toukou Souleymane, Diagana Yacouba, Moussa Diagana, Abdias Ogobara Dougnon, Antonio Vargas, and André Briend. 2021. "Effectiveness and Coverage of Treatment for Severe Acute Malnutrition Delivered by Community Health Workers in the Guidimakha Region, Mauritania" Children 8, no. 12: 1132. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121132