Improvement of Wear, Pitting Corrosion Resistance and Repassivation Ability of Mg-Based Alloys Using High Pressure Cold Sprayed (HPCS) Commercially Pure-Titanium Coatings
Abstract
:1. Introduction
2. Experimental Methods
2.1. Feedstock Powders and Substrate
2.2. CS Deposition
- CS Al coating on AZ31B Mg.
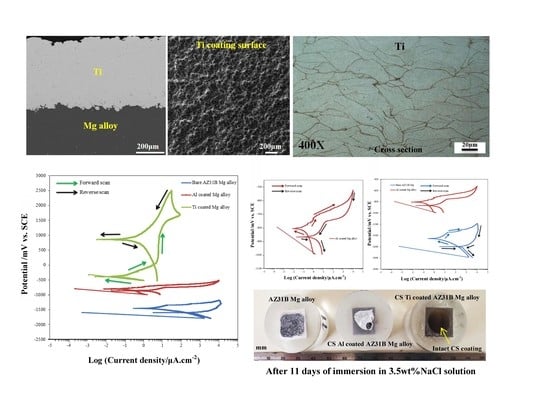
- CS Ti coating on AZ31B Mg.
2.3. Characterization
2.4. Sample Preparation for Corrosion Tests
2.5. Cyclic Potentiodynamic Polarization (CPP) Tests in 3.5 wt % NaCl Solution
2.6. Electrochemical Impedance Spectroscopy (EIS) in 3.5 wt % NaCl Solution
3. Results and Discussion
3.1. Powders Morphology and Coatings Microstructure
3.2. Electrochemical Behavior
3.2.1. Cyclic Potentiodynamic Polarization (CPP)
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. Long Term Immersion Test in 3.5 wt % NaCl Solution for 11 Days
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, A.; Du, J.; Wang, J.; Mu, S.; Zhang, G.; Li, W. Preparation and characterization of colored Ti/Zr conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2015, 276, 239–247. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Kamali, H.A. Microstructural characterization and corrosion resistance evaluation of nanostructured Al and Al/AlCr coated Mg–Zn–Ce–La alloy. Alloys Compd. 2014, 615, 657–671. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.K.; Singh, R. Exploring environment friendly nickel electrodeposition on AZ91 magnesium alloy: Effect of prior surface treatments and temperature of the bath on corrosion behavior. Corros. Sci. 2019, 151, 1–19. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.A.; Yusof, M.N.; Bakhsheshi-Rad, R.H. Fabrication and properties of triplex NiCrAlY/nano Al2O3· 13% TiO2/nano TiO2 coatings on a magnesium alloy by atmospheric plasma spraying method. Alloys Compd. 2015, 645, 450–466. [Google Scholar] [CrossRef]
- Carboneras, M.; López, M.D.; Rodrigo, P.; Campo, M.; Torres, B.; Otero, E.; Rams, J. Corrosion behaviour of thermally sprayed Al and Al/SiCp composite coatings on ZE41 magnesium alloy in chloride medium. Corros. Sci. 2010, 52, 761–768. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.; Bakhsheshi-Rad, R.H.; Yusof, M.N.; Izman, S.; Hamzah, E.; Kadir, A.M. Corrosion resistance investigation of nanostructured Si-and Si/TiO2-coated Mg alloy in 3.5% NaCl solution. Vacuum 2014, 108, 61–65. [Google Scholar]
- Bakhsheshi-Rad, R.H.; Hamzah, E.; Ebrahimi-Kahrizsangi, R.; Daroonparvar, M.; Medraj, M. Fabrication and characterization of hydrophobic micro arc oxidation/poly-lactic acid duplex coating on biodegradable Mg–Ca alloy for corrosion protection. Vacuum 2016, 125, 185–188. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.; Bakhsheshi-Rad, H.R.; Ku-mar, P.; Kay, C.M.; Kalvala, P.R. Fabrication and Corrosion Resistance Evaluation of Novel Epoxy/Oxide Layer (MgO) Coating on Mg Alloy. Prot. Met. Phys. Chem. Surf. 2020, 56, 1039–1050. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Adabi, M.; Hamzah, E.; Kamali, H.A. Improvement of corrosion resistance of binary Mg-Ca alloys using duplex alu-minum-chromium coatings. J. Mater. Eng. Perform. 2015, 7, 2614–2627. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.; Gupta, R.K.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Ghandvar, H. Investigation of Corrosion Protection Performance of Multiphase PEO (Mg2SiO4, MgO, MgAl2O4) Coatings on Mg Alloy Formed in Aluminate-Silicate- based Mixture Electrolyte. Prot. Met. Phys. Chem. Surf. 2018, 54, 425–441. [Google Scholar] [CrossRef]
- Widener, A.C.; Ellingsen, M.; Carter, M. Understanding Cold Spray for Enhanced Manufacturing Sustainability. Mater. Sci. Forum 2018, 941, 1867–1873. [Google Scholar] [CrossRef]
- Monette, Z.; Kasar, K.A.; Daroonparvar, M.; Menezes, L.P. Supersonic particle deposition as an additive technology: Methods, challenges, and applications. Int. J. Adv. Manuf. Technol. 2020, 106, 2079–2099. [Google Scholar] [CrossRef]
- Kay, C.M.; Karthikeyan, J. High Pressure Cold Spray: Principles and Applications; ASM Thermal Spray Society: Materials Park, OH, USA, 2016; ISBN 978-1-62708-096-5. [Google Scholar]
- Zulkifli, I.S.M.; Yajid, M.A.M.; Idris, M.H.; Uday, M.B.; Daroonparvar, M.; Emadzadeh, A.; Arshad, A. Micro-structural evaluation and thermal oxidation behaviors of YSZ/NiCoCrAlYTa coatings deposited by different thermal techniques. Ceram. Int. 2020, 46, 22438–22451. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Kay, C.M.M.; Bakhsheshi-Rad, H.; Gupta, R.K.; Yusof, N.M.; Ghandvar, H.; Arshad, A.; Zulkifli, I.S.M. Effects of Al2O3 diffusion barrier layer (including Y-containing small oxide precipitates) and nanostructured YSZ top coat on the oxidation behavior of HVOF NiCoCrAl-TaY/APS YSZ coatings at 1100 °C. Corros. Sci. 2018, 144, 13–34. [Google Scholar] [CrossRef]
- Luo, X.T.; Li, C.X.; Shang, F.L.; Yang, G.J.; Wang, Y.Y.; Li, C.J. High velocity impact induced microstructure evolution during deposition of cold spray coatings. Surf. Coat. Technol. 2014, 254, 11–20. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying: A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef] [Green Version]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E. Microstructural characterisation of air plasma sprayed nanostructure ceramic coatings on Mg–1% Ca alloys (bonded by NiCoCrAlYTa). Ceram. Int. 2016, 42, 357–371. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hussain, M.; Hamzah, E. Evaluation of Normal and Nanolayer Composite Thermal Barrier Coatings in Fused Vanadate-Sulfate Salts at 1000 °C. Adv. Mater. Sci. Eng. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Abdellahi, M.; Hamzah, E.; Daroonparvar, M.; Rafiei, M. Introducing a composite coating containing CNTs with good corrosion properties: Characterization and simulation. RSC Adv. 2016, 6, 108498–108512. [Google Scholar] [CrossRef]
- Wang, J.; Pang, X.; Jahed, H. Surface protection of Mg alloys in automotive applications: A review. Mater. Sci. 2019, 6, 567–600. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, T.; Sun, C.; Kong, L.; Cui, X.; Li, T.; Song, G.L. Microstructure and corrosion performance of a cold sprayed aluminium coating on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 3191–3197. [Google Scholar] [CrossRef]
- DeForce, B.S.; Eden, T.J.; Potter, J.K. Cold spray Al–5% Mg coatings for the corrosion protection of magnesium alloys. J. Therm. Spray Technol. 2011, 20, 1352–1358. [Google Scholar] [CrossRef]
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al–1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Ngai, S.; Ngai, T.; Vogel, F.; Story, W.; Thompson, G.B.; Brewer, L.N. Saltwater corrosion behavior of cold sprayed AA7075 aluminum alloy coatings. Corros. Sci. 2018, 130, 231–240. [Google Scholar] [CrossRef]
- Wei, Y.K.; Li, Y.J.; Zhang, Y.; Luo, X.T.; Li, C.J. Corrosion resistant nickel coating with strong adhesion on AZ31B magnesium alloy prepared by an in-situ shot-peening-assisted cold spray. Corros. Sci. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Han, E.H.; Xu, L. Mechanical and corrosion properties in 3.5% NaCl solution of cold sprayed Al-based coatings. Surf. Coat. Technol. 2020, 385, 125372. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium. Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, 291–302. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Cui, K. Innovative fabrication of porous titanium coating on titanium by cold spraying and vacuum sintering. Mater. Lett. 2008, 62, 3623–3625. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.; Yin, S. Solid-state cold spraying of Ti and its alloys: A literature review. Prog. Mater. Sci. 2020, 110, 100633. [Google Scholar] [CrossRef]
- Morończyk, B.; Ura-Bińczyk, E.; Kuroda, S.; Jaroszewicz, J.; Molak, R.M. Microstructure and corrosion resistance of warm sprayed titanium coatings with polymer sealing for corrosion protection of AZ91E magnesium alloy. Surf. Coat. Technol. 2019, 363, 142–151. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, B.; Wu, Z.; Qi, Z.; Wang, Z. A comparative study on the corrosion behavior of Al, Ti, Zr and Hf metallic coatings deposited on AZ91D magnesium. Surf. Coat. Technol. 2016, 303, 94–102. [Google Scholar] [CrossRef]
- ASTM E2109-01 Standard Test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings; ASTM: West Conshohocken, PA, USA, 2014.
- ASTM G133-05 Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear; ASTM: West Conshohocken, PA, USA, 2005.
- Menezes, P.L.; Nosonovsky, M.; Ingole, S.P.; Kailas, S.V.; Lovell, M.R. Tribology for Scientists and Engineers; Springer: Berlin, Germany, 2013. [Google Scholar]
- Khun, N.W.; Tan AW, Y.; Liu, E. Mechanical and tribological properties of cold sprayed Ti coatings on Ti-6Al-4V substrates. J. Therm. Spray Technol. 2016, 25, 715–724. [Google Scholar] [CrossRef]
- Siddique, S.; Li, C.X.; Bernussi, A.A.; Hussain, S.W.; Yasir, M. Enhanced Electrochemical and Tribo-logical Properties of AZ91D Mg Alloy via Cold Spraying of Al Alloy. J. Therm. Spray Technol. 2019, 28, 1739–1748. [Google Scholar] [CrossRef]
- ASTM Standard G61-86 Standard Test Method for Conducting Cyclic Potentiodynamic Polarization (CPP) Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys; ASTM: West Conshohocken, PA, USA, 2014.
- Cizek, J.; Kovarik, O.; Siegl, J.; Khor, K.A.; Dlouhy, I. Influence of plasma and cold spray deposited titanium Layers on high-cycle fatigue properties of Ti6Al4V substrates. Surf. Coat. Technol. 2013, 217, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Tan, A.W.Y.; Marinescu, I.; Toh, W.Q.; Liu, E. Adhesion, tribological and corrosion properties of cold-sprayed CoCrMo and Ti6Al4V coatings on 6061-T651 Al alloy. Surf. Coat. Technol. 2017, 326, 291–298. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.Y.; Khun, N.W.; Marinescu, I.; Liu, E. Effect of substrate surface condition on fatigue behavior of cold sprayed Ti6Al4V coatings. Surf. Coat. Technol. 2017, 320, 452–457. [Google Scholar] [CrossRef]
- Astarita, A.; Rubino, F.; Carlone, P.; Ruggiero, A.; Leone, C.; Genna, S.; Merola, M.; Squillace, A. On the Improvement of AA2024 Wear Properties through the Deposition of a Cold-Sprayed Titanium Coating. Metals 2016, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, G.Z.; Cheng, Y.F. Electronic structure and pitting behavior of 3003 aluminum alloy passivated under various conditions. Electrochim. Acta 2009, 54, 4155–4163. [Google Scholar] [CrossRef]
- Kumar, S.; Jyothirmayi, A.; Wasekar, N.; Joshi, S.V. Influence of annealing on mechanical and electrochemical properties of cold sprayed niobium coatings. Surf. Coat. Technol. 2016, 296, 124–135. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Moreto, J.A.; Marino, C.E.B.; Bose Filho, W.W.; Rocha, L.A.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behavior of high strength Al and Al–Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]
- Kendig, M.W.; Mansfeld, F. AC electrochemical impedance of a model pit. J. Electrochem. Soc. 1982, 129, C318. [Google Scholar]
- Guo, F.; Jiang, W.; Tang, G.; Xie, Z.; Dai, H.; Wang, E.; Chen, Y.; Liu, L. Enhancing anti-wear and anti-corrosion performance of cold spraying aluminum coating by high current pulsed electron beam irradiation. Vacuum 2020, 182, 109772. [Google Scholar] [CrossRef]
- Da Silva, F.S.; Bedoya, J.; Dosta, S.; Cinca, N.; Cano, I.G.; Guilemany, J.M.; Benedetti, A.V. Corrosion characteristics of cold gas spray coatings of reinforced aluminum deposited onto carbon steel. Corros. Sci. 2017, 114, 57–71. [Google Scholar] [CrossRef] [Green Version]
- López-Ortega, A.; Arana, J.L.; Rodríguez, E.; Bayón, R. Corrosion, wear and tribo-corrosion performance of a thermally sprayed aluminum coating modified by plasma electrolytic oxidation technique for offshore submerged components protection. Corros. Sci. 2018, 143, 258–280. [Google Scholar] [CrossRef]
- Lu, F.F.; Ma, K.; Li, C.X.; Yasir, M.; Luo, X.T.; Li, C.J. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Technol. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Cao, C.N.; Lin, H.; Lu, M. Electrochemical potential noise of 321 stainless steel stressed under constant strain rate testing conditions. Electrochim. Acta 2007, 52, 2123–2133. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Liu, L.; Liu, S.; Hou, B.; Liu, Q.; Ding, H. Effect of ultrasonic cold forging technology as the pretreatment on the corrosion resistance of MAO Ca/P coating on AZ31B Mg alloy. J. Alloys Compd. 2015, 635, 278–288. [Google Scholar]
- Liu, M.; Mao, X.; Zhu, H.; Lin, A.; Wang, D. Water and corrosion resistance of epoxy–acrylic–amine waterborne coatings: Effects of resin molecular weight, polar group and hydrophobic segment. Corros. Sci. 2013, 75, 106–113. [Google Scholar]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg–1%Ca alloy formed in aluminate electrolyte containing titania nano-additives. Alloys Compd. 2016, 68, 841–857. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Mardanikivi, T. Deposition of duplex MAO layer/nanostructured titanium dioxide composite coatings on Mg–1% Ca alloy using a combined technique of air plasma spraying and micro arc oxidation. Alloys Compd. 2015, 649, 591–605. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Zhong, X.; Zhang, L.; Chen, B. Composite anticorrosion coatings for AZ91D magnesium alloy with molybdate conversion coating and silicon sol–gel coatings. Prog. Org. Coat. 2009, 66, 199–205. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fang, H.; Hang, C.; Sun, Y.; Peng, Z.; Wei, W.; Wang, Y. Fabrication and characterization of silk fibroin coating on APTES pretreated Mg-Zn-Ca alloy. Mater. Sci. Eng. C 2020, 110, 110742. [Google Scholar] [CrossRef]
- Merl, D.K.; Panjan, P.; Kovač, J. Corrosion and surface study of sputtered Al–W coatings with a range of tungsten contents. Corros. Sci. 2013, 69, 359–368. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Khan, M.U.F.; Saadeh, Y.; Kay, C.M.; Kasar, A.K.; Kumar, P.; Esteves, L.; Misra, M.; Menezes, P.; Kalvala, P.R. Modification of surface hardness, wear re-sistance and corrosion resistance of cold spray Al coated AZ31B Mg alloy using cold spray double layered Ta/Ti coating in 3.5wt % NaCl solution. Corros. Sci. 2020, 176, 109029. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication corrosion resistance of a hydrophobic micro- arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Farooq, A.; Hamza, M.; Ahmed, Q.; Deen, K.M. Evaluating the performance of zinc and aluminum sacrificial anodes in artificial seawater. Electrochim. Acta 2019, 314, 135–141. [Google Scholar] [CrossRef]
- Cui, L.Y.; Gao, S.D.; Li, P.P.; Zeng, R.C.; Zhang, F.; Li, S.Q.; Han, E.H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]














| Alloy Name | Mg | Al | Zn | Si | Mn | Fe | Cu | Ca | Ni | C |
|---|---|---|---|---|---|---|---|---|---|---|
| AZ31B Mg alloy | Balance | 2.8 | 1.1 | <0.08 | 0.7 | <0.01 | <0.01 | <0.03 | <0.001 | – |
| Propellant Gas | Sprayed Material | Gas Temperature (C°) | Gas Pressure (MPa) | Spray Angle (°) | Stand-Off Distance (mm) | Step Size (mm) | Powder Feed Rate (RPM) | Powder Carrier Gas Flow Rate (m3/hr) | Type of Nozzle |
|---|---|---|---|---|---|---|---|---|---|
| N2 | CP-Al | 350–600 | 3.0–5.0 | 90° | 25.4 | 0.508 | 2.0–3.5 | 3.0 | SiC water cooled nozzle |
| N2 | CP-Ti | 700–1050 | 3.0–5.0 | 90° | 25.4 | 1.016 | 2.0–3.5 | 3.0 | SiC water cooled nozzle |
| Type of Sample | Ecorr (mVSCE) | βa (mV/dec) | βc (mV/dec) | icorr (μA cm−2) |
|---|---|---|---|---|
| AZ31B | −1453.86 | 115.3 | 67.6 | 2.504 |
| Al-coated AZ31B | −804.59 | 75.5 | 114 | 0.092 |
| Ti-coated AZ31B | −387.299 | 159.9 | 99.7 | 0.049 |
| Samples | Rs (Ω cm2) | Rct (Ω cm2) | Qdl (F cm−2sn–1) | n | Rpo (Ω cm2) | Qc (F cm−2sn–1) | n | RL (Ω cm2) | L (H cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Bare AZ31B Mg alloy | 18.31 | 385.6 | 1.13 × 10−5 | 0.98 | - | - | - | 187.9 | 187.5 |
| Al-coated Mg alloy | 15.18 | 164707 | 0.116 × 10−3 | 0.87 | 14397 | 9.68 × 10−6 | 0.88 | - | - |
| Ti-coated Mg alloy | 17.69 | 5.58 × 106 | 66.67 × 10−6 | 0.798 | 6059 | 59.33 × 10−6 | 0.90 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daroonparvar, M.; Kasar, A.K.; Farooq Khan, M.U.; L. Menezes, P.; Kay, C.M.; Misra, M.; Gupta, R.K. Improvement of Wear, Pitting Corrosion Resistance and Repassivation Ability of Mg-Based Alloys Using High Pressure Cold Sprayed (HPCS) Commercially Pure-Titanium Coatings. Coatings 2021, 11, 57. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11010057
Daroonparvar M, Kasar AK, Farooq Khan MU, L. Menezes P, Kay CM, Misra M, Gupta RK. Improvement of Wear, Pitting Corrosion Resistance and Repassivation Ability of Mg-Based Alloys Using High Pressure Cold Sprayed (HPCS) Commercially Pure-Titanium Coatings. Coatings. 2021; 11(1):57. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11010057
Chicago/Turabian StyleDaroonparvar, Mohammadreza, Ashish K. Kasar, Mohammad Umar Farooq Khan, Pradeep L. Menezes, Charles M. Kay, Manoranjan Misra, and Rajeev K. Gupta. 2021. "Improvement of Wear, Pitting Corrosion Resistance and Repassivation Ability of Mg-Based Alloys Using High Pressure Cold Sprayed (HPCS) Commercially Pure-Titanium Coatings" Coatings 11, no. 1: 57. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11010057