Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Polymer Synthesis
2.3. Preparation of Oxygen Barrier Coatings
2.4. Characterization
2.4.1. Morphology of Coatings
2.4.2. Oxygen Permeability of Coating
2.4.3. Structure of the Coating
2.4.4. Curing Mechanism Analysis
2.4.5. Thermogravimetric Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of MMA-MAc-DAM-MA Copolymer
3.2. Effect of Curing Temperature on the Barrier Performance of Coated PET Film
3.3. Analysis of Cross-Linking Structure of Curing Coating
3.4. DSC Analysis of Coatings Cured at Different Temperatures
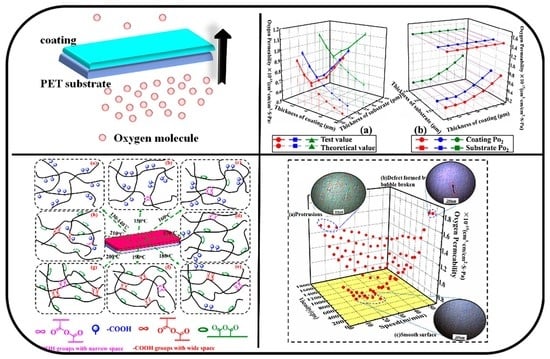
3.5. The Effect of Coating Thicknesson Oxygen Barrier Property of Coated Film
3.6. The Effect of Coating Solution Viscosity and Coating Speed on Barrier Property of Coated PET Film
3.7. The Optimal Coating Process Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mchugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modifiedprocedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 2010, 58, 899–903. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.H.; Kim, H.W.; Ham, J.S. Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Tenn, N.; Follain, N.; Fatyeyeva, K.; Poncin-Epaillard, F.; Labrugère, C.; Marais, S. Impact of hydrophobic plasma treatments on the barrier properties of poly(lactic acid) films. RSC Adv. 2014, 4, 5626. [Google Scholar] [CrossRef]
- Yang, X.; Martinson, A.B.F.; Jeffrey, W.E.; Shao, L.; Darling, S.B. Water treatment based on atomically engineered materials: Atomic layer deposition and beyond. Matter 2021, 4, 3515–3548. [Google Scholar] [CrossRef]
- Saito, A.; Obata, S.; Nishina, Y. Uniform coating of magnesium oxide crystal with reduced graphene oxide achieves moisture barrier performance. Appl. Surf. Sci. 2022, 573, 151483. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, S.; Ter-Ovanessian, B.; Hermange, K.; Huo, Y.; Normand, B. Enhancing the barrier effect of sol-gel derived inorganic coating by doping h-BN nanosheet. Appl. Surf. Sci. 2021, 544, 148849. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. VII. A free volume-based model with mechanical relaxation. J. Appl. Polym. Sci. 1987, 33, 533–549. [Google Scholar] [CrossRef]
- Vieth, W.R.; Sladek, K.J. A model for diffusion in a glassy polymer. J. Colloid Sci. 1965, 20, 1014–1033. [Google Scholar] [CrossRef]
- Müller-Plathe, F.; Rogers, S.C.; van Gunsteren, W.F. Gas sorption and transport in polyisobutylene: Equilibrium and nonequilibrium molecular dynamics simulations. J. Chem. Phys. 1993, 98, 9895–9904. [Google Scholar] [CrossRef]
- Wang, J.; Pan, T.; Zhang, J.; Xu, X.; Yin, Q.; Han, J.; Wei, M. Hybrid films with excellent oxygen and water vapor barrier properties as efficient anticorrosive coatings. RSC Adv. 2018, 8, 21651–21657. [Google Scholar] [CrossRef] [Green Version]
- Stergioudi, F.; Baxevani, A.; Mavropoulos, A.; Skordaris, G. Mechanical properties and diffusion barrier performance of CrWNcoatings fabricated through hybrid HiPIMS/RFMS. Coatings 2021, 11, 690. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Chen, J.-B.; Li, X.-J.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Innovative enhancement of gas barrier properties of biodegradable poly(butylene succinate) nanocomposite films by introducing confined crystals. RSC Adv. 2016, 6, 2530–2536. [Google Scholar] [CrossRef]
- Scully, A.D.; Mao, Q.; Fell, C.J. High-performance oxygen barrier inorganic-organic coating for polymeric substrates. Surf. Coat. Technol. 2014, 239, 222–226. [Google Scholar] [CrossRef]
- Haile, M.; Sarwar, O.; Henderson, R.; Smith, R.; Grunlan, J.C. Polyelectrolyte coacervates deposited as high gas barrier thin films. Macromol. Rapid Commun. 2017, 38, 1600594. [Google Scholar] [CrossRef] [Green Version]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer—solvent systems. I. Reexamination of the free-volume theory. J. Polym. Sci. Pol. Phys. 1977, 15, 403–416. [Google Scholar] [CrossRef]
- Heuchel, M.; Fritsch, D.; Budd, P.M.; McKeown, N.B.; Hofmann, D. Atomistic packing model and free volume distribution of a polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2008, 318, 84–99. [Google Scholar] [CrossRef]
- Takeuchi, H.; Roe, R.J.; Mark, J.E. Molecular dynamics simulation of diffusion of small molecules in polymers. II. Effect of free volume distribution. J. Chem. Phys. 1990, 93, 9042–9048. [Google Scholar] [CrossRef]
- Lau, C.H.; Li, P.; Li, F.; Chung, T.S.; Paul, D.R. Reverse-selective polymeric membranes for gas separations. Prog. Polym. Sci. 2013, 38, 740–766. [Google Scholar] [CrossRef]
- Hagen, D.A.; Box, C.; Greenlee, S.; Xiang, F.; Regev, O.; Grunlan, J.C. High gas barrier imparted by similarly charged multilayers in nanobrick wall thin films. RSC Adv. 2014, 4, 18354–18359. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Konstas, K.; Doherty, C.M.; Wood, C.D.; Mulet, X.; Xie, Z.; Ng, D.; Hill, M.R.; Shao, L.; Lau, C.H. Hyper-cross-linked additives that impede aging and enhance permeability in thin polyacetylene films for organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 14401–14408. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H. A jump motion of small molecules in glassy polymers: A molecular dynamics simulation. J. Chem. Phys. 1990, 93, 2062–2067. [Google Scholar] [CrossRef]
- Hofmann, D.; Fritz, L.; Ulbrich, J.; Paul, D. Molecular simulation of small molecule diffusion and solution in dense amorphous polysiloxanes and polyimides. Comput. Theor. Polym. Sci. 2000, 10, 419–436. [Google Scholar] [CrossRef]
- Müller-Plathe, F. Molecular dynamics simulation of gas transport in amorphous polypropylene. J. Chem. Phys. 1992, 96, 3200–3205. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Siepmann, J.; Gpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef]
- Nakaya, M.; Yasuhara, S.; Maeda, T.; Hotta, A. Impact of hot wire and material gas species on the Cat-CVD coating of gas barrier SiOC thin films onto PET bottles. Surf. Coat. Technol. 2018, 344, 21–29. [Google Scholar] [CrossRef]
- Angelo Miranda, M.; Jabarin, S.A.; Coleman, M. Modification of poly(ethylene terephthalate) (PET) using linoleic acid for oxygen barrier improvement: Impact of processing methods. J. Appl. Polym. Sci. 2017, 134, 45023. [Google Scholar] [CrossRef]
- Wei, J.; Peng, S.; Xue, B.; Yang, Z.; Qin, S.; Yu, J.; Xu, G. Effect of silane functionalized graphene prepared by a supercritical carbon dioxide process on the barrier properties of polyethylene terephthalate composite films. RSC Adv. 2019, 9, 21903–21910. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, J.; Ma, J. Synthesis of a cationic fluorinated polyacrylate emulsifier-free emulsion via ab initio RAFT emulsion polymerization and its hydrophobic properties of coating films. RSC Adv. 2015, 5, 97231–97238. [Google Scholar] [CrossRef]
- Azpitarte, I.; Botta, G.A.; Tollan, C.; Knez, M. SCIP: A new simultaneous vapor phase coating and infiltration process for tougher and UV-resistant polymer fibers. RSC Adv. 2020, 10, 15976–15982. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Qiu, G.; Ting, Y.P.; Chung, T.S. Silver-PEGylated dendrimer nanocomposite coating for anti-fouling thin film composite membranes for water treatment. Colloids Surf. A 2013, 436, 207–214. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, Y.; Zhang, S.; Liu, M.; Li, X.; Cai, T. Hyperbranchedpoly(ionic liquid) functionalized poly(ether sulfone) membranes as healable antifouling coatings for osmotic power generation. J. Mater. Chem. A 2019, 7, 8167–8176. [Google Scholar] [CrossRef]
- Kornilova, N.; Bikbulatova, A.; Koksharov, S.; Aleeva, S.; Radchenko, O.; Nikiforova, E. Multifunctional polymer coatings of fusible interlinings for sewing products. Coatings 2021, 11, 616. [Google Scholar] [CrossRef]
- Somorjai, G.A. Molecular level studies of solid-gas and solid-liquid interfaces. Surf. Sci. 1995, 335, 10–22. [Google Scholar] [CrossRef]
- Idris, A.; Muntean, A.; Mesic, B.; Lestelius, M.; Javed, A. Oxygen barrier performance of poly(vinyl alcohol) coating films with different induced crystallin. Coatings 2021, 11, 1253. [Google Scholar] [CrossRef]
- Liu, B.; Huang, X.; Wang, S.; Wang, D.; Guo, H. Performance of polyvinyl alcohol/bagasse fibre foamed composites as cushion packaging materials. Coatings 2021, 11, 1094. [Google Scholar] [CrossRef]
- Amano, N.; Takahashi, M.; Uchiyama, H.; Kozuka, H. Transferability and adhesion of sol–gel-derived crystalline tio2 thin films to different types of plastic substrates. Langmuir 2017, 33, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, X.; Chen, K.; Lee, K.N. Sintering modeling of thermal barrier coatings at elevated temperatures: A review of recent advances. Coatings 2021, 21, 1214. [Google Scholar] [CrossRef]
- Dhieb, F.B.; Dil, E.J.; Tabatabaei, S.H.; Mighri, F.; Ajji, A. Effect of nanoclay orientation on oxygen barrier properties of LbL nanocomposite coated films. RSC Adv. 2019, 9, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Sindhu, S.; Varman, K.A.; Ramamurthy, P.C.; Madras, G. Hybrid nanocomposite films of polyvinyl alcohol and ZnO as interactive gas barrier layers for electronics device passivation. RSC Adv. 2012, 2, 11536. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Chen, J.; Zhang, W.-Q.; Ji, X.; Li, Z.-M. High barrier graphene oxide nanosheet/poly(vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409–410, 156–163. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, M.H.; Ko, J.A.; Kang, D.H.; Bae, H.; Park, H.J. Influence of food with high moisture content on oxygen barrier property of polyvinyl alcohol (PVA)/vermiculite nanocomposite coated multilayer packaging film. J. Food Sci. 2018, 83, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Budd, P.M.; Price, C.; Satgurunathan, R. The permeability of poly(butyl acrylate)/poly(vinylidene chloride-stat-acrylonitrile) core/shell emulsion polymers for use as gas barrier coatings. Eur. Polym. J. 1993, 29, 337–342. [Google Scholar] [CrossRef]
- Delafresnaye, L.; Dugas, P.Y.; Dufils, P.E.; Chaduc, I.; Vinas, J.M.; Lansalot, M.; Bourgeat-Lami, E. Synthesis of clay-armored poly(vinylidene chloride-co-methyl acrylate) latexes by Pickering emulsion polymerization and their film-forming properties. Polym. Chem. 2017, 8, 6217–6232. [Google Scholar] [CrossRef]
- Gamier, J.; Dufils, P.E.; Vinas, J.; Vanderveken, Y.; Herk, A.V.; Lacroix-Desmazes, P. Synthesis of poly(vinylidene chloride)-based composite latexes by emulsion polymerization from epoxy functional seeds for improved thermal stability. Polym. Degrad. Stab. 2012, 97, 170–177. [Google Scholar] [CrossRef]
- Hwang, T.; Pu, L.; Kim, S.W.; Oh, Y.S.; Nam, J.D. Synthesis and barrier properties of poly(vinylidene chloride-co-acrylonitrile)/SiO2 hybrid composites by sol–gel process. J. Membr. Sci. 2016, 345, 90–96. [Google Scholar] [CrossRef]
- Kodani, T.; Sakai, H.; Okabe, T.; Nomura, M. Effect of vinylidene chloride content on film-formation property of vinylidene chloride-methyl acrylate copolymer latex. J. Appl. Polym. Sci. 2015, 69, 565–572. [Google Scholar] [CrossRef]
- Collins, S.; Yoda, K.; Anazawa, N.; Birkinshaw, C. Thermal stability of some vinylidene chloride copolymers. Polym. Degrad. Stab. 1999, 66, 87–94. [Google Scholar] [CrossRef]
- Wu, M.S.; Painter, P.C.; Coleman, M.M. Further studies of the preferred chain conformation of crystalline poly(vinylidene chloride). J. Polym. Sci. Pol. Phys. Ed. 1980, 18, 95–110. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, H.; Li, B.; Li, B. Mechanism and kinetics of emulsion copolymerization of vinylidene chloride--Critical conversion at the end of interval 2 and rate of polymerization. Polym. Int. 2010, 30, 441–444. [Google Scholar] [CrossRef]
- Velasquez, E.; Rieger, J.; Stoffelbach, F.; d’Agosto, F.; Lansalot, M.; Dufils, P.E.; Vinas, J. Surfactant-free poly(vinylidene chloride) latexes via one-pot RAFT-mediated aqueous polymerization. Polymer 2016, 106, 275–284. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Belhaneche-Bensemra, N.; Gelbard, G. Stabilizing effect of epoxidized sunflower oil on the thermal degradation of poly(vinyl chloride). Polym. Degrad. Stab. 2001, 74, 501–505. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Shao, Q.; Pan, D.; Wujick, E.; Guo, J.; Xie, X.; Xie, X.; Guo, Z. Crosslinkednorbornene copolymer anion exchange membrane for fuel cells. J. Membr. Sci. 2018, 556, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Ge, S.; Wang, J.; Zhang, X.; Zhang, T.; Lin, J.; Zhao, C.X.; Wang, B.; Zhu, G.; Guo, Z. Waterborne acrylic resin modified with glycidyl methacrylate (GMA): Formula optimization and property analysis. Polymer 2018, 143, 155–163. [Google Scholar] [CrossRef]
- Lu, M.; Huang, S.; Chen, S.; Ju, Q.; Xiao, M.; Peng, X.; Wang, S.; Meng, Y. Transparent and super-gas-barrier PET film with surface coated by a polyelectrolyte and Borax. Polym. J. 2018, 50, 239–250. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Song, E.; Jo, K.; Yun, T.; Moon, M.-W.; Lee, K.-R. Composite oxygen-barrier coating on a polypropylene food container. Thin Solid Films 2013, 540, 112–117. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Yang, X.; Sun, J.; Gao, H.; Bai, Y.; Shao, L. Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property. Coatings 2021, 11, 1451. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11121451
Zhong W, Yang X, Sun J, Gao H, Bai Y, Shao L. Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property. Coatings. 2021; 11(12):1451. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11121451
Chicago/Turabian StyleZhong, Wen, Xiaobin Yang, Jikun Sun, Hongwei Gao, Yongping Bai, and Lu Shao. 2021. "Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property" Coatings 11, no. 12: 1451. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11121451