Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
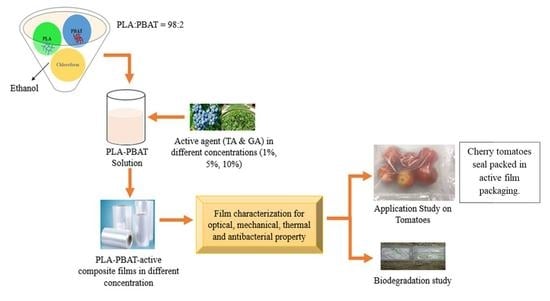
2.2. Preparation of Composite Films with Efficient Polyphenols
2.3. Film Characterization
2.3.1. Morphological Observation
2.3.2. Surface Colour and Optical Properties
2.3.3. Thickness and Mechanical Properties
2.3.4. Thermal Stability
2.3.5. Surface Hydrophobicity
2.3.6. Water Vapour Permeability
2.3.7. Antibacterial Activity
2.4. Effect of Developed Composite Films on Cherry Tomato Quality
2.5. Total Bacterial Count
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Blend Films
3.1.1. Morphology of the Surface
3.1.2. Surface Colour
3.1.3. Optical Properties
3.1.4. Mechanical Properties
3.1.5. Water Contact Angle (WCA)
3.1.6. Water Vapour Permeability
3.1.7. Thermogravimetric Analysis
3.1.8. Antibacterial Activity
3.2. Effects of Composite Films on the Cherry Tomato Quality
3.2.1. Weight Loss
3.2.2. Total Soluble Solids and pH
3.2.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur Food Res Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R.; Akashi, M. Ferulic acid-coupled chitosan: Thermal stability and utilization as an antioxidant for biodegradable active packaging film. Carbohydr. Polym. 2015, 115, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Tueen, B.S. Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Han, J.H. Antimicrobial food packaging. In Novel Food Packaging Techniques; Woodhead Publishing: Sawston, UK, 2003; Volume 8, pp. 50–70. [Google Scholar]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Kutlu, N.; Meral, R.; Ekin, M.M.; Kose, Y.E.; Ceylan, Z. A new application for the valorisation of pomegranate seed oil: Nanoencapsulation of pomegranate seed oil into electrospun nanomats for food preservation. Int. J. Food Sci. Technol. 2022, 57, 1074–1082. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of phenolic compounds for food preservation: Food additive and active packaging. In Phenolic Compounds–Biological Activity; IntechOpen: London, UK, 2017. [Google Scholar]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Rhim, J.-W.; Hong, S.-I. Preparation of poly(lactide)/poly(butylene adipate-co-terephthalate) blend films using a solvent casting method and their food packaging application. LWT Food Sci. Technol. 2016, 68, 454–461. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide) /poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Zhen, Z.; Ying, S.; Hongye, F.; Jie, R.; Tianbin, R. Preparation, Characterization and Properties of Binary and Ternary Blends with Thermoplastic Starch, Poly(Lactic Acid) and Poly(Butylene Succinate). Polym. Renew. Resour. 2011, 2, 49–62. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of antibacterial poly(lactide)/poly(butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Owczarek, A.; Nadolna, K.; Sionkowska, A. The film-forming properties of chitosan with tannic acid addition. Mater. Lett. 2019, 245, 22–24. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Wang, Y.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. Double Cross-Linked Chitosan Composite Films Developed with Oxidized Tannic Acid and Ferric Ions Exhibit High Strength and Excellent Water Resistance. Biomacromolecules 2019, 20, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Aragüez, L.; Colombo, A.; Borneo, R.; Aguirre, A. Active packaging from triticale flour films for prolonging storage life of cherry tomato. Food Packag. Shelf Life 2020, 25, 100520. [Google Scholar] [CrossRef]
- ISO 6887-1: 1999; Microbiology of food and animal feeding stuffs — Preparation of test samples, initial suspension and decimal dilutions for microbiological examination — Part 1: General rules for the preparation of the initial suspension and decimal dilutions. International Standards of Organisation: Geneva, Switzerland, 1999.
- Huang, D.; Zhang, Z.; Quan, Q.; Zheng, Y. Tannic acid: A versatile and effective modifier for gelatin/zein composite films. Food Packag. Shelf Life 2020, 23, 100440. [Google Scholar] [CrossRef]
- Ahn, B.J.; Gaikwad, K.K.; Lee, Y.S. Characterization and properties of LDPE film with gallic-acid-based oxygen scavenging system useful as a functional packaging material. J. Appl. Polym. Sci. 2016, 133, 44138. [Google Scholar] [CrossRef]
- Goudar, N.; Vanjeri, V.N.; Dixit, S.; Hiremani, V.; Sataraddi, S.; Gasti, T.; Vootla, S.K.; Masti, S.P.; Chougale, R.B. Evaluation of multifunctional properties of gallic acid crosslinked Poly (vinyl alcohol)/Tragacanth Gum blend films for food packaging applications. Int. J. Biol. Macromol. 2020, 158, 139–149. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Mulla, M.; Ahmed, J.; Al-Attar, H.; Castro-Aguirre, E.; Arfat, Y.A.; Auras, R. Antimicrobial efficacy of clove essential oil infused into chemically modified LLDPE film for chicken meat packaging. Food Control 2017, 73, 663–671. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L. Developing a bio-based packaging film from soya by-products incorporated with valonea tannin. J. Clean Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Annual book of ASTM Standard; ASTM D882-88: Standard Methods for Tensile Properties of Thin Plastic Sheeting; American Society for Testing & Materials: Philadelphia, PA, USA, 1989; Volume 8, pp. 519–836.
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P.; Salami, E.; Fearday, C. Effects of heat treatment on chitosan nanocomposite film reinforced with nanocrystalline cellulose and tannic acid. Carbohydr. Polym. 2016, 140, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The antimicrobial, mechanical, physical and structural properties of chitosan–gallic acid films. LWT Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Şen, F.; Uzunsoy, İ.; Baştürk, E.; Kahraman, M.V. Antimicrobial agent-free hybrid cationic starch/sodium alginate polyelectrolyte films for food packaging materials. Carbohydr. Polym. 2017, 170, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef] [PubMed]
- Thiyagu, T.T.; JV, S.P.K.; Sathiyamoorthy, V.; VR, A.P. Effect of cashew shell biomass synthesized cardanol oil green compatibilizer on flexibility, barrier, thermal, and wettability of PLA/PBAT biocomposite films. Biomass Convers. Biorefin. 2021, 1–11. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Kim, H.C.; Pham, D.H.; Alrobei, H.; Kim, J. Tannic-Acid-Cross-Linked and TiO2-Nanoparticle-Reinforced Chitosan-Based Nanocomposite Film. Polymers 2021, 13, 228. [Google Scholar] [CrossRef]
- Pyla, R.; Kim, T.-J.; Silva, J.L.; Jung, Y.-S. Enhanced antimicrobial activity of starch-based film impregnated with thermally processed tannic acid, a strong antioxidant. Int. J. Food Microbiol. 2010, 137, 154–160. [Google Scholar] [CrossRef]
- Widsten, P.; Mesic, B.B.; Cruz, C.D.; Fletcher, G.C.; Chycka, M.A. Inhibition of foodborne bacteria by antibacterial coatings printed onto food packaging films. J. Food Sci. Technol. 2017, 54, 2379–2386. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. LWT 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Buendía, L.; Soto, S.; Ros, M.; Antolinos, V.; Navarro, L.; Sánchez, M.J.; Martínez, G.B.; López, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]






| Film | L | A | B | ΔE | UV Barrier at T(280) | Transparency at T(600) |
|---|---|---|---|---|---|---|
| PLA/PBAT | 93.06 ± 0.01 a | −0.31 ± 0.01 d | 2.34 ± 0.01 a | 4.38 ± 0.03 c | 2.33 ± 0.03 d | 73.38 ± 0.15 c |
| PLA/PBAT-TA1% | 93.81 ± 0.02 b | −0.61 ± 0.01 c | 3.41 ± 0.01 b | 3.97 ± 0.01 b | 1.71 ± 0.06 a | 58.41 ± 0.22 a |
| PLA/PBAT-TA5% | 94.07 ± 0.01 c | −0.69 ± 0.01 b | 3.58 ± 0.01 c | 3.87 ± 0.00 a | 1.58 ± 0.07 b | 55.63 ± 0.16 b |
| PLA/PBAT-TA10% | 94.28 ± 0.03 d | −0.73 ± 0.01 a | 3.73 ± 0.01 d | 3.88 ± 0.01 a | 1.45 ± 0.02 c | 52.83 ± 0.05 b |
| PLA/PBAT-GA1% | 93.68 ± 0.02 b | −0.56 ± 0.01 c | 3.37 ± 0.01 b | 3.83 ± 0.01 a | 1.85 ± 0.07 a | 52.16 ± 0.12 b |
| PLA/PBAT-GA5% | 94.07 ± 0.05 c | −0.67 ± 0.00 b | 3.56 ± 0.01 c | 3.84 ± 0.00 a | 1.69 ± 0.12 b | 48.68 ± 0.06 a |
| PLA/PBAT-GA10% | 94.25 ± 0.01 d | −0.86 ± 0.01 a | 3.90 ± 0.01 d | 3.92 ± 0.01 b | 1.53 ± 0.03 c | 45.17 ± 0.03 d |
| Film | Thickness (µm) | Tensile Strength (TS) (MPa) | Elongation at Break (EB) (%) | Elastic Modulus (EM) (MPa) | Water Contact Angle (WCA) |
|---|---|---|---|---|---|
| PLA/PBAT | 55.53 ± 0.03 a | 4.80 ± 0.06 a | 21.94 ± 11.42 b | 199.80 ± 8.15 c | 73.91° ± 2.79 a |
| PLA/PBAT-TA1% | 55.52 ± 0.03 a | 5.21 ± 0.85 a | 21.98 ± 16.32 c | 165.41 ± 6.32 a | 73.15° ± 0.50 ab |
| PLA/PBAT-TA5% | 56.02 ± 0.06 b | 6.37± 0.08 b | 22.38 ± 11.51 a | 172.68 ± 5.13 ab | 76.01° ± 3.48 ab |
| PLA/PBAT-TA10% | 56.43 ± 0.02 b | 8.63 ± 0.3 c | 23.52 ± 9.18 b | 181.56 ± 3.17 b | 77.94° ± 0.78 b |
| PLA/PBAT-GA1% | 56.19 ± 0.01 b | 4.98 ± 0.51 a | 23.10 ± 19.01 c | 168.21 ± 6.64 b | 72.51° ± 1.07 b |
| PLA/PBAT-GA5% | 57.43 ± 0.02 c | 5.97 ± 0.08 b | 22.42 ± 16.43 a | 177.46 ± 9.97 a | 72.64° ± 1.07 b |
| PLA/PBAT-GA10% | 58.51 ± 0.01 d | 7.01 ± 0.95 c | 22.09 ± 18.64 a | 189.71± 16.04 c | 68.34° ± 1.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Perera, K.Y.; Pradhan, D.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato. Coatings 2022, 12, 1902. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12121902
Sharma S, Perera KY, Pradhan D, Duffy B, Jaiswal AK, Jaiswal S. Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato. Coatings. 2022; 12(12):1902. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12121902
Chicago/Turabian StyleSharma, Shubham, Kalpani Y. Perera, Dileswar Pradhan, Brendan Duffy, Amit K. Jaiswal, and Swarna Jaiswal. 2022. "Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato" Coatings 12, no. 12: 1902. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12121902