Simple UV-Grafting of PolyAcrylic and PolyMethacrylic Acid on Silicone Breast Implant Surfaces: Chemical and Mechanical Characterizations
Abstract
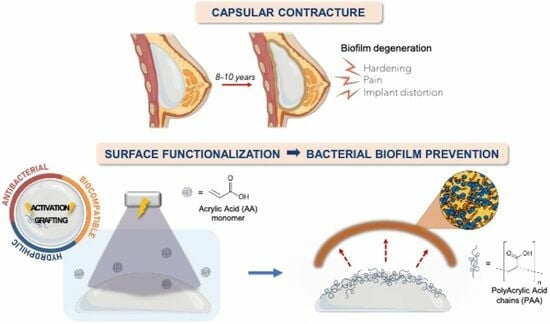
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization Process
2.3. Surface Characterizations
3. Results and Discussion
3.1. Direct Grafting of PMAc and PAAc
3.2. Direct Grafting with pH Middle Adjustment
3.3. Mechanical Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Chiappone, A.; Dietliker, K.; Pirri, C.F.; Roppolo, I. Fabrication and Functionalization of 3D Printed Polydimethylsiloxane-Based Microfluidic Devices Obtained through Digital Light Processing. Adv. Mater. Technol. 2020, 5, 2000374. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A.-A. Modification of Polysiloxane Polymers for Biomedical Applications: A Review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Kottegoda, N.; Ratnaweera, D.R.; Weerasekera, M.M. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega 2021, 6, 11488–11496. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.A.; Santos, C.; Monge, N.; Santos, N.C.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Using Plasma-Mediated Covalent Functionalization of Rhamnolipids on Polydimethylsiloxane towards the Antimicrobial Improvement of Catheter Surfaces. Biomater. Adv. 2022, 134, 112563. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of Silicone Surface Modification Techniques and Coatings for Antibacterial/Antimicrobial Applications to Improve Breast Implant Surfaces. Acta Biomater. 2021, 121, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Pileri, S.A.; Pravettoni, G.; Viale, G.; De Lorenzi, F.; Nolè, F.; Veronesi, P.; Curigliano, G. Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Comprehensive Review. Cancer Treat. Rev. 2020, 84, 101963. [Google Scholar] [CrossRef] [PubMed]
- Moris, V.; Lam, M.; Amoureux, L.; Magallon, A.; Guilloteau, A.; Maldiney, T.; Zwetyenga, N.; Falentin-Daudre, C.; Neuwirth, C. What Is the Best Technic to Dislodge Staphylococcus Epidermidis Biofilm on Medical Implants? BMC Microbiol. 2022, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, B.H.; Yoo, B.Y.; Nam, S.; Lee, M.; Choi, J.; Park, H.; Choy, Y.B.; Heo, C.Y.; Koh, W. Modulation of Foreign Body Reaction against PDMS Implant by Grafting Topographically Different Poly(Acrylic Acid) Micropatterns. Macromol. Biosci. 2019, 19, 1900206. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.H.; Kim, B.H.; Kim, S.; Lee, K.; Choy, Y.B.; Heo, C.Y. Silicone Breast Implant Modification Review: Overcoming Capsular Contracture. Biomater. Res. 2018, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Bayston, R. Capsule Formation around Breast Implants. JPRAS Open 2022, 31, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Shah, N.; Clemens, M.W.; Horwitz, S.M. How I Treat Breast Implant–Associated Anaplastic Large Cell Lymphoma. Blood 2018, 132, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhu, S.; Ishihara, K.; Brash, J.L. Adsorption of Fibrinogen and Lysozyme on Silicon Grafted with Poly(2-Methacryloyloxyethyl Phosphorylcholine) via Surface-Initiated Atom Transfer Radical Polymerization. Langmuir 2005, 21, 5980–5987. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Vayron, R.; Delille, R.; Migonney, V.; Falentin-Daudré, C. Influence of Poly(Styrene Sodium Sulfonate) Grafted Silicone Breast Implant’s Surface on the Biological Response and Its Mechanical Properties. Mater. Today Commun. 2022, 31, 103318. [Google Scholar] [CrossRef]
- Chouirfa, H.; Evans, M.D.M.; Bean, P.; Saleh-Mghir, A.; Crémieux, A.C.; Castner, D.G.; Falentin-Daudré, C.; Migonney, V. Grafting of Bioactive Polymers with Various Architectures: A Versatile Tool for Preparing Antibacterial Infection and Biocompatible Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Sommerfeld, S.D.; Murthy, N.S.; Kohn, J.; Migonney, V. Poly(NaSS) Functionalization Modulates the Conformation of Fibronectin and Collagen Type I To Enhance Osteoblastic Cell Attachment onto Ti6Al4V. Langmuir 2014, 30, 9477–9483. [Google Scholar] [CrossRef]
- Mehdi, A.S.; Bitar, G.; Sharma, R.K.; Iyengar, S.; El-Sharkawi, D.; Tasoulis, M.K.; Attygalle, A.D.; Cunningham, D.; Sharma, B. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): A Good Practice Guide, Pictorial Review, and New Perspectives. Clin. Radiol. 2022, 77, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Köllnberger, A.; Schrader, R.; Briehn, C.A. Carboxylic Acid Mediated Antimicrobial Activity of Silicone Elastomers. Mater. Sci. Eng. C 2020, 113, 111001. [Google Scholar] [CrossRef]
- Braem, A.; Kamarudin, N.H.N.; Bhaskar, N.; Hadzhieva, Z.; Mele, A.; Soulié, J.; Linklater, D.P.; Bonilla-Gameros, L.; Boccaccini, A.R.; Roy, I.; et al. Biomaterial Strategies to Combat Implant Infections: New Perspectives to Old Challenges. Int. Mater. Rev. 2023, 1–39. [Google Scholar] [CrossRef]
- Askari, F.; Zandi, M.; Shokrolahi, P.; Tabatabaei, M.H.; Hajirasoliha, E. Reduction in Protein Absorption on Ophthalmic Lenses by PEGDA Bulk Modification of Silicone Acrylate-Based Formulation. Prog. Biomater. 2019, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric Sustainable Materials and Their Emerging Applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Michiardi, A.; Hélary, G.; Nguyen, P.-C.T.; Gamble, L.J.; Anagnostou, F.; Castner, D.G.; Migonney, V. Bioactive Polymer Grafting onto Titanium Alloy Surfaces. Acta Biomater. 2010, 6, 667–675. [Google Scholar] [CrossRef]
- Pereira, C.; Baumann, J.-S.; Humblot, V.; Falentin-Daudré, C. Biological Properties of Direct Grafting by Ultraviolet Irradiation of Vinyl Benzyl Phosphonic Acid onto Titanium Surfaces. React. Funct. Polym. 2022, 173, 105215. [Google Scholar] [CrossRef]
- Chouirfa, H.; Migonney, V.; Falentin-Daudré, C. Grafting Bioactive Polymers onto Titanium Implants by UV Irradiation. RSC Adv. 2016, 6, 13766–13771. [Google Scholar] [CrossRef]
- Amokrane, G.; Falentin-Daudré, C.; Ramtani, S.; Migonney, V. A Simple Method to Functionalize PCL Surface by Grafting Bioactive Polymers Using UV Irradiation. IRBM 2018, 39, 268–278. [Google Scholar] [CrossRef]
- Yammine, P.; Pavon-Djavid, G.; Helary, G.; Migonney, V. Surface Modification of Silicone Intraocular Implants To Inhibit Cell Proliferation. Biomacromolecules 2005, 6, 2630–2637. [Google Scholar] [CrossRef]
- Migonney, V.; Lacroix, M.D.; Ratner, B.D.; Jozefowicz, M. Silicone Derivatives for Contact Lenses: Functionalization, Chemical Characterization, and Cell Compatibility Assessment. J. Biomater. Sci. Polym. Ed. 1996, 7, 265–275. [Google Scholar] [CrossRef]
- Crémieux, A.-C.; Pavon-Djavid, G.; Mghir, A.S.; Hélary, G.; Migonney, V. Bioactive Polymers Grafted on Silicone to Prevent Staphylococcus Aureus Prosthesis Adherence: In Vitro and in VIVO Studies. J. Appl. Biomater. Biomech. 2003, 1, 178–185. [Google Scholar]
- Lam, M.; Falentin-Daudré, C. Implication of the Nature and Texturation of Silicone Surfaces on the Grafting of PolyNaSS, a Bioactive Polymer. IRBM 2022, 43, 687–693. [Google Scholar] [CrossRef]
- Lam, M.; Moris, V.; Humblot, V.; Migonney, V.; Falentin-Daudre, C. A Simple Way to Graft a Bioactive Polymer—Polystyrene Sodium Sulfonate on Silicone Surfaces. Eur. Polym. J. 2020, 128, 109608. [Google Scholar] [CrossRef]
- Lam, M.; Falentin-Daudré, C. Characterization of Plasmatic Proteins Adsorption on Poly(Styrene Sodium Sulfonate) Functionalized Silicone Surfaces. Biophys. Chem. 2022, 285, 106804. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.M.; Falentin-Daudré, C.; Blanquaert, D.; Thomas, D.; Granja, P.L.; Migonney, V. Role of Protein Environment and Bioactive Polymer Grafting in the S. Epidermidis Response to Titanium Alloy for Biomedical Applications. Mater. Sci. Eng. C 2014, 45, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Poussard, L.; Ouédraogo, C.P.; Pavon-Djavid, G.; Migonney, V. Inhibition de l’adhérence de Staphylococcus epidermidis sur des surfaces en titane par des polymères hydrosolubles bioactifs comportant des fonctions sulfonate, phosphate ou carboxylate. Pathol. Biol. 2012, 60, 84–90. [Google Scholar] [CrossRef]
- De Giglio, E.; Cometa, S.; Cioffi, N.; Torsi, L.; Sabbatini, L. Analytical Investigations of Poly(Acrylic Acid) Coatings Electrodeposited on Titanium-Based Implants: A Versatile Approach to Biocompatibility Enhancement. Anal. Bioanal. Chem. 2007, 389, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hou, Z. Homogenous Grafted Poly(Acrylic Acid) Brushes on Ultra-Flat Polydimethlysiloxane (PDMS) Films by UV Irradiation. Nano BioMed. Eng. 2011, 3, 42–46. [Google Scholar] [CrossRef]
- Velazco-Medel, M.A.; Camacho-Cruz, L.A.; Bucio, E. Modification of PDMS with Acrylic Acid and Acrylic Acid/Ethylene Glycol Dimethacrylate by Simultaneous Polymerization Assisted by Gamma Radiation. Radiat. Phys. Chem. 2020, 171, 108754. [Google Scholar] [CrossRef]
- Schneider, M.H.; Willaime, H.; Tran, Y.; Rezgui, F.; Tabeling, P. Wettability Patterning by UV-Initiated Graft Polymerization of Poly(Acrylic Acid) in Closed Microfluidic Systems of Complex Geometry. Anal. Chem. 2010, 82, 8848–8855. [Google Scholar] [CrossRef]
- Zuñiga-Zamorano, I.; Meléndez-Ortiz, H.I.; Costoya, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Poly(Vinyl Chloride) Catheters Modified with pH-Responsive Poly(Methacrylic Acid) with Affinity for Antimicrobial Agents. Radiat. Phys. Chem. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, P.P.; Mondal, S.; Das, P.; Bhawal, P.; Dhara, S.; Das, N.C. Polysaccharide and Poly(Methacrylic Acid) Based Biodegradable Elastomeric Biocompatible Semi-IPN Hydrogel for Controlled Drug Delivery. Mater. Sci. Eng. C 2018, 92, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, Y.; Wu, Y.; Xiong, L.; Hang, T.; Ling, H.; Hu, A.; Gao, L.; Li, M. Grafting of Poly(Methacrylic Acid-Co-Acrylamide) Film on Silicon Surface via a Simultaneous Hydrolysis Process. Mater. Today Commun. 2019, 21, 100678. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive Manufacturing of Sustainable Biomaterials for Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.N.; Dong, R.; Ober, C.K.; Baird, B.A. Cellular Responses to Patterned Poly(Acrylic Acid) Brushes. Langmuir 2011, 27, 7016–7023. [Google Scholar] [CrossRef]
- Salloum, D.S.; Schlenoff, J.B. Protein Adsorption Modalities on Polyelectrolyte Multilayers. Biomacromolecules 2004, 5, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Baker, G.L.; Bruening, M.L. Use of Porous Membranes Modified with Polyelectrolyte Multilayers as Substrates for Protein Arrays with Low Nonspecific Adsorption. Anal. Chem. 2006, 78, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ikada, Y. Selective Adsorption of Proteins to Their Ligands Covalently Immobilized onto Microfibers. Biotechnol. Bioeng. 1995, 47, 557–566. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-sections AT 1254 And 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Frueh, J.; Gai, M.; Yang, Z.; He, Q. Influence of Polyelectrolyte Multilayer Coating on the Degree and Type of Biofouling in Freshwater Environment. J. Nanosci. Nanotech. 2014, 14, 4341–4350. [Google Scholar] [CrossRef]
- Frueh, J.; Reiter, G.; Möhwald, H.; He, Q.; Krastev, R. Orientation Change of Polyelectrolytes in Linearly Elongated Polyelectrolyte Multilayer Measured by Polarized UV Spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 366–373. [Google Scholar] [CrossRef]
- Park, D.; Wang, J.; Klibanov, A.M. One-Step, Painting-Like Coating Procedures To Make Surfaces Highly and Permanently Bactericidal. Biotechnol. Prog. 2006, 22, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.C.; Lynn, R.A.P.; Hearn, J.; Paul, A.J.; Vickerman, J.C.; Watts, J.F. Surface Chemical Characterization Using XPS and ToF-SIMS of Latex Particles Prepared by the Emulsion Copolymerization of Methacrylic Acid and Styrene. Langmuir 1996, 12, 3866–3875. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Available online: https://www.iso.org/obp/ui/fr/#iso:std:iso:14607:ed-3:v2:en (accessed on 5 October 2022).












| Atome | Position * (eV) | %at. Non-Grafted | %at. PMAc Grafted | %at. PAAc Grafted |
|---|---|---|---|---|
| C1s | 283.8 | 49.1 ± 2.2 | 54.0 ± 2.3 | 51.1 ± 2.5 |
| O1s | 531.3 | 23.0 ± 0.3 | 23.8 ± 0.3 | 24.4 ± 1.2 |
| Si2p | 101.3 | 27.9 ± 2.1 | 21.8 ± 2.0 | 24.5 ± 3.7 |
| C1s | O1s | Si2p | C288/Ctotal | O533/Ototal | ||||
|---|---|---|---|---|---|---|---|---|
| Assignment | C-C, C-H | C-O, Cα | C=O | C=O(OH) O-Si | C=O(OH) | Si-O | ||
| PDMS | 283.8 | 531.3 | 101.2 | |||||
| 47.6 | 22.7 | 29.7 | ||||||
| PAAc | 283.6 | 284.4 | 288.3 | 531.1 | 532.6 | 101.1 | ||
| 36.25 | 12.45 | 5.6 | 20.9 | 5.1 | 19.8 | 0.103 | 0.196 | |
| PMAc | 283.8 | 284.8 | 288.3 | 531.3 | 532.8 | 101.3 | ||
| 43.0 | 9.0 | 4.1 | 19.5 | 3.9 | 20.4 | 0.073 | 0.166 | |
| Atome | Position * (eV) | %at. Non Grafted | %at. PAAc Grafted | %at. PAAc-pH Grafted |
|---|---|---|---|---|
| C1s | 283 | 49.1 ± 2.2 | 51.1 ± 2.5 | 52.3 ± 0.9 |
| O1s | 531 | 23.0 ± 0.3 | 24.4 ± 1.2 | 28.5 ± 0.3 |
| Si2p | 101 | 27.9 ± 2.1 | 24.5 ± 3.7 | 19.2 ± 1.3 |
| Non-Grafted | PAAc Grafted | PAAc-pH Grafted | |
|---|---|---|---|
| Young modulus (MPa) | 2.07 ± 0.16 | 2.77 ± 0.29 | 1.71 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniak, A.; Humblot, V.; Vayron, R.; Delille, R.; Falentin-Daudré, C. Simple UV-Grafting of PolyAcrylic and PolyMethacrylic Acid on Silicone Breast Implant Surfaces: Chemical and Mechanical Characterizations. Coatings 2023, 13, 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111888
Wozniak A, Humblot V, Vayron R, Delille R, Falentin-Daudré C. Simple UV-Grafting of PolyAcrylic and PolyMethacrylic Acid on Silicone Breast Implant Surfaces: Chemical and Mechanical Characterizations. Coatings. 2023; 13(11):1888. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111888
Chicago/Turabian StyleWozniak, Anna, Vincent Humblot, Romain Vayron, Rémi Delille, and Céline Falentin-Daudré. 2023. "Simple UV-Grafting of PolyAcrylic and PolyMethacrylic Acid on Silicone Breast Implant Surfaces: Chemical and Mechanical Characterizations" Coatings 13, no. 11: 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13111888