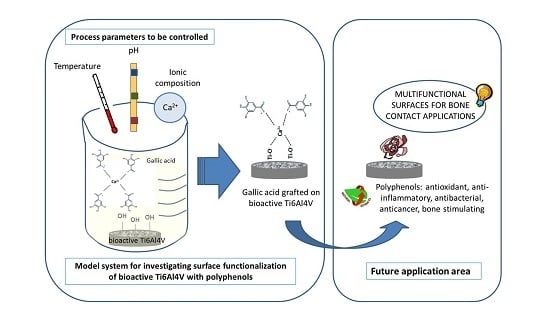
Grafting of Gallic Acid onto a Bioactive Ti6Al4V Alloy: A Physico-Chemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Activation
2.2. Surface Functionalization
2.3. Detection of the Grafted Biomolecule
2.4. Contact Angle Measurements
2.5. In Vitro Apatite-Forming Ability Tests (Bioactive Behavior)
2.6. Field Emission Scanning Electron Microscopy (FESEM) Observations and Energy Dispersive Spectroscopy (EDS) Analyses
2.7. Electro-Kinetic Measurements
3. Results and Discussion
3.1. pH Measurements
3.2. Biomolecule Detection
3.3. Contact Angle Measurements
3.4. In Vitro Apatite-Forming Ability Tests
3.5. Zeta Potential Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- El Gharras, H. Polyphenols: food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Dosier, C.R.; Erdman, C.P.; Park, J.H.; Schwartz, Z.; Boyan, B.D.; Guldberg, R.E. Resveratrol effect on osteogenic differentiation of rat and human adipose derived stem cells in a 3-D culture environment. J. Mech. Behav. Biomed. Mater. 2012, 11, 112–122. [Google Scholar] [CrossRef]
- Li, Y.; Bäckesjö, C.M.; Haldosén, L.A.; Lindgren, U. Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur. J. Pharmacol. 2009, 609, 13–18. [Google Scholar] [CrossRef]
- Đudarić, L.; Fužinac-Smojver, A.; Muhvić, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yuan, H.; Cheng, L.; Zhou, X.; Huang, X.; Li, J. Effects of gallic acid on the morphology and growth of hydroxyapatite crystals. Arch. Oral Biol. 2015, 60, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Si, S.; Zhang, Q. Water-dispersible hydroxyapatite nanoparticles synthesized in aqueous solution containing grape seed extract. Appl. Surf. Sci. 2012, 258, 3578–3583. [Google Scholar] [CrossRef]
- Saikia, J.P.; Konwarh, R.; Konwar, B.K.; Karak, N. Isolation and immobilization of Aroid polyphenol on magnetic nanoparticles: Enhancement of potency on surface immobilization. Colloids Surf. B 2013, 102, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, Y.; Li, H.; Li, M.; Zhang, D.; Huixia, F.; Feng, H.; Fun, H. Dual-functionalization based on combination of quercetin compound and rare earth nanoparticle. J. Rare. Earths 2013, 31, 709–714. [Google Scholar] [CrossRef]
- Mohanty, R.K.; Thennarasu, S.; Mandal, A.B. Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surf. B 2014, 114, 138–143. [Google Scholar] [CrossRef]
- Li, Y.; Dånmark, S.; Edlund, U.; Finne-Wistrand, A.; He, X.; Norgård, M.; Blomén, E.; Hultenby, K.; Andersson, G.; Lindgren, U. Resveratrol-conjugated poly-e-caprolactone facilitates in vitro mineralization and in vivo bone regeneration. Acta Biomater. 2011, 7, 751–758. [Google Scholar] [CrossRef]
- Neo, Y.P.; Swift, S.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.; Perera, C.O. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013, 141, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ambrosi, M.; Caldera, F.; Trotta, F.; Berrueta, L.; Gallo, B. Encapsulation of apple polyphenols in β-CD nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 85–92. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a useful vector for rutin topical formulations: Synthesis, characterization and testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, Y.D.; Vedala, H.; Allen, B.L.; Star, A. Exploring the chemical sensitivity of a carbon nanotube/green tea composite. Acs Nano 2010, 4, 6854–6862. [Google Scholar] [CrossRef]
- Sousa, F.; Guebitz, G.M.; Kokol, V. Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process Biochem. 2009, 44, 749–756. [Google Scholar] [CrossRef]
- Božič, M.; Gorgieva, S.; Kokol, V. Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr. Polym. 2012, 89, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Nunesa, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Trifković, K.T.; Milašinović, N.Z.; Djordjević, V.B.; Kruši, M.T.K.; Knežević-Jugović, Z.D.; Nedović, V.A.; Bugarski, B.M. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2014, 111, 901–907. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Stojanović, R.; Manojlović, V.; Komes, D.; Cindrić, I.J.; Nedović, V.; Bugarski, B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate–chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res. Int. 2011, 44, 1094–1101. [Google Scholar] [CrossRef]
- Nagarajan, S.; Rami Reddy, B.S.; Tsibouklis, J. In vitro effect on cancer cells: Synthesis and preparation of polyurethane membranes for controlled delivery of curcumin. J. Biomed. Mater. Res. Part A 2011, 99, 410–417. [Google Scholar] [CrossRef]
- Wu, H.; Wu, C.; He, Q.; Liao, X.; Shi, B. Collagen fiber with surface-grafted polyphenol as a novel support for Pd(0) nanoparticles: Synthesis, characterization and catalytic application. Mater. Sci. Eng. C 2010, 30, 770–776. [Google Scholar] [CrossRef]
- Seshadri, S.; Sastry, T.P.; Jeevitha, D.; Samiksha, N. Synthesis and characterization of a novel bone graft material containing biphasic calcium phosphate and chitosan fortified with aloe vera. Int. J. Drug Regul. Aff. 2014, 2, 85–90. [Google Scholar]
- Lin, F.H.; Dong, G.C.; Chen, K.S.; Jiang, G.J.; Huang, C.W.; Sun, J.S. Immobilization of Chinese herbal medicine onto the surface-modified calcium hydrogenphosphate. Biomaterials 2003, 24, 2413–2422. [Google Scholar] [CrossRef] [Green Version]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Zhang, X.; Prenesti, E.; Corazzari, I.; Turci, F.; Tomatis, M.; Vernè, E. Gallic acid grafting to a ferrimagnetic bioactive glass-ceramic. J. Non-Cryst. Solids 2016, 432, 167–175. [Google Scholar] [CrossRef]
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E.; Ferraris, S. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Bianchi, C.L.; Cassinelli, C.; Vernè, E. Surface modification of Ti-6Al-4 V alloy for biomineralization and specific biological response: Part II, alkaline phosphatase grafting. J. Mater. Sci. Mater. Med. 2011, 22, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cell. Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.; Anandan, C.; Rajendran, N. Drug release characteristics of quercetin-loaded TiO2 nanotubes coated with chitosan. Int. J. Boil. Macromol. 2016, 93, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; Gonzales-Martin, M.L.; Monjo, M.; Ramis, J.M. Flavonoid modified surfaces: Multifunctional bioactive biomaterials with osteopromotive, anti-inflammatory and anti-fibrotic potential. Adv. Heathc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gurzawska, K.; Svava, R.; Yihua, Y.; Haugshøj, K.B.; Dirscherl, K.; Levery, S.B.; Byg, I.; Damager, I.; Nielsen, M.W.; Jørgensen, B.; Jørgensen, N.R.; Gotfredsen, K. Osteoblastic response to pectin nanocoating on titanium surfaces. Mater. Sci. Eng. C 2014, 43, 117–125. [Google Scholar] [CrossRef]
- Erakovic, S.; Jankovic, A.; Tsui, G.; Tang, C.Y.; Miskovic-Stankovic, V.; Stevanovic, T. Novel bioactive antimicrobial lignin containing coatings on titanium obtained by electrophoretic deposition. Int. J. Mol. Sci. 2014, 15, 12294–12322. [Google Scholar] [CrossRef]
- Džunuzović, E.S.; Džunuzović, J.V.; Marinković, A.D.; Marinović-Cincović, M.T.; Jeremić, K.B.; Nedeljković, J.M. Influence of surface modified TiO2 nanoparticles by gallates on the properties of PMMA/TiO2 nanocomposites. Eur. Polym. J. 2012, 48, 1385–1393. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2003, 35, 473–485. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- ASTM B348 Standard Specification for Titanium and Titanium Alloy Bars and Billets; ASTM: West Conshohocken, PA, USA, 2010.
- Spriano, S.; Vernè, E.; Ferraris, S. Multifunctional Titanium Surfaces for Bone Integration. EP Patent 2,214,732, 11 August 2010. [Google Scholar]
- Ferraris, S.; Spriano, S.; Pan, G.; Venturello, A.; Bianchi, C.L.; Chiesa, R.; Faga, M.G.; Maina, G.; Vernè, E. Surface modification of Ti–6Al–4V alloy for biomineralization and specific biological response: Part I, inorganic modification. J. Mater. Sci. Mater. Med. 2011, 22, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterals 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Surleva, A.; Atanasova, P.; Kolusheva, T.; Costadinnova, L. Study of the complex equilibrium between titanium (IV) and tannic acid. J. Chem. Technol. Metall. 2014, 49, 594–600. [Google Scholar]
- Huguenin, J.; Hamady, S.O.; Bourson, P. Monitoring deprotonation of gallic acid by Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 1062–1066. [Google Scholar] [CrossRef]
- Mera, A.C.; Contreras, D.; Escalona, N.; Mansilla, H.D. BiOI microspheres for photocatalytic degradation of gallic acid. J. Photochem. Photobiol. A 2016, 318, 71–76. [Google Scholar] [CrossRef]
- Araujo, P.Z.; Morando, P.J.; Blesa, M.A. Interaction of catechol and gallic acid with titanium dioxide in aqueous suspensions 1. Equilibrium studies. Langmuir 2005, 21, 3470–3474. [Google Scholar] [CrossRef]
- Ball, V.; Meyer, F. Deposition kinetics and electrochemical properties of tannic acid on gold and silica. Colloids Surf. A 2016, 491, 12–17. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Buzzone, G.; Carpi, A.; DiSanti, G.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int. J. Oral Maxillofac. Implant. 2003, 18, 40–45. [Google Scholar]
- Prajatelistia, E.; Ju, S.W.; Sanandiya, N.D.; Jun, S.H.; Ahn, J.S.; Hwang, D.S. Tunicate-inspired gallic acid/metal ion complex for instant and efficient treatment of dentin hypersensitivity. Adv. Healthc. Mater. 2016, 5, 919–927. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, J.; Wang, X.; Wang, J.; Huang, N. Inspired chemistry for a simple but highly effective immobilization of vascular endothelial growth factor on GA functionalized plasma polymerized film Plasma Process. Plasma Process. Polym. 2012, 9, 718–725. [Google Scholar] [CrossRef]
- Qiao, G.; Su, J.; He, M. Effect of (–)-epigallocatechin gallate on electrochemical behavior and surface film composition of Co–Cr alloy used in dental restorations. Dent. Mater. J. 2012, 31, 564–574. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Rose, K.D.; Kwiatek, P.J. Carbon aromaticity based on XPS II to II* signal intensity. Appl. Surf. Sci. 1993, 64, 167–174. [Google Scholar] [CrossRef]
- Öteyaka, M.Ö.; Chevallier, P.; Robitaillec, L.; Larocheb, G. Effect of surface modification by ammonia plasma on vascular graft: PET film and PET scaffold. Acta Phys. Pol. A 2012, 121, 125–127. [Google Scholar] [CrossRef]
- Ferraris, S.; Venturello, A.; Miola, M.; Cochis, A.; Rimondini, L.; Spriano, S. Antibacterial and bioactive nanostructured titanium surfaces for bone integration. Appl. Surf. Sci. 2014, 311, 279–291. [Google Scholar] [CrossRef]
- Ferraris, S.; Bobbio, A.; Miola, M.; Spriano, S. Micro- and nano-textured, hydrophilic and bioactive titanium dental implants. Surf. Coat. Technol. 2015, 276, 374–383. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B 2015, 129, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Luxbacher, T. The ZETA Guide: Principles of the Streaming Potential Technique; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Oc’wieja, M.; Adamczyk, Z.; Morga, M. Adsorption of tannic acid on polyelectrolyte monolayers determined in situ by streaming potential measurements. J. Colloid Interface Sci. 2015, 438, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, A.; Aurousseau, M.; Guillet, A.; Mauret, E. Effect of pH and ionic strength on the electrical charge and particle size distribution of starch nanocrystal suspensions. Starch-Stärke 2015, 67, 319–327. [Google Scholar] [CrossRef]
- Botelho, C.M.; LopesI, M.A.; Gibson, R.; Best, S.M.; Santos, J.D. Structural analysis of Si-substituted hydroxyapatite: Zeta potential and X-ray photoelectron spectroscopy. J. Mater. Sci. Mater. Med. 2002, 13, 1123–1127. [Google Scholar] [CrossRef] [PubMed]








| Sample Acronym | Sample Description |
|---|---|
| WATER + GA_1 | Solution 1 mg/mL of Gallic acid in ultrapure water |
| WATER + GA_2 | Solution 2 mg/mL of Gallic acid in ultrapure water |
| SBF + GA_1 | Solution 1 mg/mL of Gallic acid in SBF |
| SBF + GA_2 | Solution 2 mg/mL of Gallic acid in SBF |
| PBS + GA_1 | Solution 1 mg/mL of Gallic acid in PBS |
| PBS + GA_2 | Solution 2 mg/mL of Gallic acid in PBS |
| CT | Ti6Al4V sample chemical treated and UV irradiated |
| CT_GA_W_1 | CT sample functionalized with solution 1 mg/mL of Gallic acid in ultrapure water |
| CT_GA_W_2 | CT sample functionalized with solution 2 mg/mL of Gallic acid in ultrapure water |
| CT_GA_SBF_1 | CT sample functionalized with solution 1 mg/mL of Gallic acid in SBF |
| CT_GA_SBF_2 | CT sample functionalized with solution 2 mg/mL of Gallic acid in SBF |
| CT_GA_PBS_1 | CT sample functionalized with solution 1 mg/mL of Gallic acid in PBS |
| CT_GA_PBS_2 | CT sample functionalized with solution 2 mg/mL of Gallic acid in PBS |
| Solution | pH | Color of the Solution |
|---|---|---|
| WATER + GA_1 (Source) | 3.35 ± 0.04 | Colorless |
| WATER + GA_2 (Source) | 3.17 ± 0.04 | Colorless |
| SBF + GA_1 (Source) | 7.45 ± 0.10 | Blue |
| SBF + GA_2 (Source) | 7.10 ±0.11 | Blue |
| PBS + GA_1 (Source) | 6.17 ±0.09 | Colorless |
| PBS + GA_2 (Source) | 4.60 ±0.12 | Colorless |
| WATER + GA_1 (Uptake) | 3.36 ± 0.01 | Colorless |
| WATER + GA_2 (Uptake) | 3.15 ± 0.04 | Light yellow |
| SBF + GA_1 (Uptake) | 7.22 ± 0.04 | Blue |
| SBF + GA_2 (Uptake) | 6.71 ± 0.08 | Blue |
| PBS + GA_1 (Uptake) | 6.05 ± 0.07 | Light yellow |
| PBS + GA_2 (Uptake) | 4.49 ± 0.04 | Colorless |
| Elements [at.%] | Samples | ||||||
|---|---|---|---|---|---|---|---|
| CT | CT_GA_W_1 | CT_GA_W_2 | CT_GA_PBS_1 | CT_GA_PBS_2 | CT_GA_SBF_1 | CT_GA_SBF_2 | |
| O | 57.0 | 52.6 | 49.6 | 59.0 | 55.5 | 44.7 | 47.8 |
| C | 19.0 | 29.9 | 31.8 | 19.1 | 20.2 | 45.2 | 39.1 |
| Ti | 18.2 | 15.9 | 14.9 | 15.6 | 15.6 | 6.6 | 8.7 |
| Ca | – | – | – | 0.8 | – | 3.5 | 3.2 |
| Others | 5.8 | 1.6 | 3.8 | – | 5.4 | – | 8.7 |
| Elements (at.%) | Samples | |
|---|---|---|
| CT | CT_GA_SBF_1 | |
| C | 7.54 ± 3.06 | 7.59 ± 2.96 |
| O | 55.10 ± 10.85 | 55.09 ± 10.86 |
| Na | 0.48 ± 0.03 | 0.45 ± 0.03 |
| Mg | 0.73 ± 0.07 | 0.75 ± 0.07 |
| P | 13.32 ± 3.54 | 12.71 ± 3.37 |
| Ca | 22.18 ± 9.68 | 22.18 ± 9.67 |
| Ti | 0.78 ± 0.42 | 1.25 ± 0.67 |
| Ca/P ratio | 1.63 ± 0.3 | 1.7 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzola, M.; Ferraris, S.; Prenesti, E.; Casalegno, V.; Spriano, S. Grafting of Gallic Acid onto a Bioactive Ti6Al4V Alloy: A Physico-Chemical Characterization. Coatings 2019, 9, 302. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9050302
Cazzola M, Ferraris S, Prenesti E, Casalegno V, Spriano S. Grafting of Gallic Acid onto a Bioactive Ti6Al4V Alloy: A Physico-Chemical Characterization. Coatings. 2019; 9(5):302. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9050302
Chicago/Turabian StyleCazzola, Martina, Sara Ferraris, Enrico Prenesti, Valentina Casalegno, and Silvia Spriano. 2019. "Grafting of Gallic Acid onto a Bioactive Ti6Al4V Alloy: A Physico-Chemical Characterization" Coatings 9, no. 5: 302. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9050302