Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Whey Protein Isolates Nanofibers (WPNFs)
2.2.1. Preparation of WPNFs
2.2.2. Transmission Electron Microscopy (TEM)
2.3. Preparation and Functional Properties of Edible Coatings (ECs) Emulsions
2.3.1. Preparation of Edible Coating Emulsions
2.3.2. Morphology of the Edible Coating Emulsions
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.4. Antioxidant Activity of the Edible Coating Emulsions
2.4. Preparation and Functional Properties of Edible Films (EFs)
2.4.1. Preparation of Edible Films
2.4.2. Antimicrobial Activity of the EFs
2.4.3. Physical Properties of Edible Films
2.5. Coated Cheese Quality Assessment
2.5.1. Preparation of Cheese Samples and Treatments
2.5.2. Evaluation of Physicochemical Properties of Coated Cheese
2.6. Statistical Analysis
3. Results and Discussion
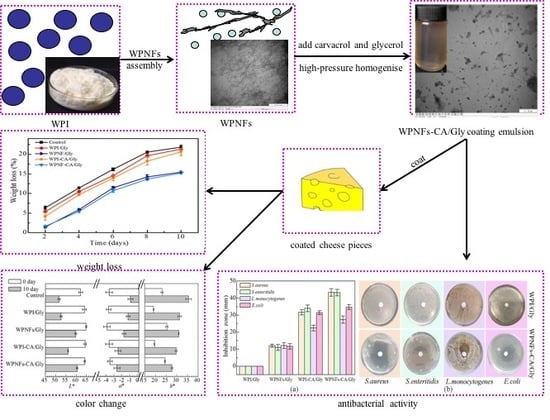
3.1. Morphology of Whey Protein Isolates Nanofibers (WPNFs)
3.2. Functional Properties of Edible Coating Emulsions
3.2.1. Appearance and Morphology of Edible Coating Emulsions
3.2.2. FT-IR of Edible Coating Emulsions
3.2.3. Antioxidant Activity of Edible Coating Emulsions
3.3. Functional Properties of Edible Films (EFs)
3.3.1. Antibacterial Activity of EFs
3.3.2. Microstructure of EFs
3.3.3. Film Thickness
3.3.4. Transparency of EFs
3.3.5. Moisture Content of EFs
3.4. Functional Properties of the Edible Coatings (ECs) on the Preservation of Cheese Pieces
3.4.1. Weight Loss of Coated Cheese Slice
3.4.2. Texture Property of Coated Cheese
3.4.3. Color Changes of the Cheese Pieces
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are building blocks of heat-induced fibrillar protein aggregates of β-lactoglobulin formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Feng, Z.; Li, D. Aggregation of Whey Protein Hydrolysate Using Alcalase 2.4 L. PLoS ONE 2014, 9, e109439. [Google Scholar] [CrossRef]
- Veerman, C.; de Schiffart, G.; Sagis, L.M.C.; van der Linden, E. Irreversible self-assembly of ovalbumin into fibrils and the resulting network rheology. Int. J. Biol. Macromol. 2003, 33, 121–127. [Google Scholar] [CrossRef]
- Akkermans, C.; Van der Goot, A.J.; Venema, P.; Gruppen, H.; Vereijken, J.M.; Van der Linden, E.; Boom, R.M. Micrometer-Sized Fibrillar Protein Aggregates from Soy Glycinin and Soy Protein Isolate. Agric. Food Chem. 2007, 55, 9877–9882. [Google Scholar] [CrossRef]
- Munialo, C.D.; Martin, A.H.; van der Linden, E.; de Jongh, H.H. Fibril formation from pea protein and subsequent gel formation. J. Agric. Food Chem. 2014, 62, 2418–2427. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation. Foods 2019, 8, 34. [Google Scholar] [CrossRef]
- Ng, S.K.; Nyam, K.L.; Nehdi, I.A.; Chong, G.H.; Lai, O.M.; Tan, C.P. Impact of stirring speed on beta-lactoglobulin fibril formation. Food Sci. Biotechnol. 2016, 25 (Suppl. 1), 15–21. [Google Scholar] [CrossRef]
- Mantovani, R.A.; de Figueiredo Furtado, G.; Netto, F.M.; Cunha, R.L. Assessing the potential of whey protein fibril as emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Moayedzadeh, S.; Madadlou, A.; Asl, A.K. Formation mechanisms, handling and digestibility of food protein nanofibrils. Trends Food Sci. Technol. 2015, 45, 50–59. [Google Scholar] [CrossRef]
- Kroes-Nijboer, A.; Venema, P.; van der Linden, E. Fibrillar structures in food. Food Funct. 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Jones, O.G.; Mezzenga, R. Inhibiting, promoting, and preserving stability of functional protein fibrils. Soft Matter 2012, 8, 876–895. [Google Scholar] [CrossRef]
- Amagliani, L.; Schmitt, C. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- Chaparro-Hernández, S.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ocaño-Higuera, V.M.; Valenzuela-López, C.C.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural Antimicrobial/Antioxidant Agents in Meat and Poultry Products as Well as Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2016, 58, 1–26. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating based on Whey Protein Nanofibrils with TiO(2) Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, J.; Ma, J.; Yuan, G.; Sun, H. Effect of chitosan-carvacrol coating on the quality of Pacific white shrimp during iced storage as affected by caprylic acid. Int. J. Biol. Macromol. 2018, 106, 123–129. [Google Scholar] [CrossRef]
- Santos, A.R.; da Silva, A.F.; Amaral, V.C.; Ribeiro, A.B.; de Abreu Filho, B.A.; Mikcha, J.M. Application of edible coating with starch and carvacrol in minimally processed pumpkin. J. Food Sci. Technol. 2016, 53, 1975–1983. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.M.C.; van der Linden, E. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Zhang, Y.; Li, X.; Liu, C.; Jiang, B.; Xu, J.; Sun, Z. Formation of Whey Protein Isolate Nanofibrils by Endoproteinase GluC and Their Emulsifying Properties. Food Hydrocoll. 2019, 94, 71–79. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Vuilliomenet, A.-M.; Rouvet, M.; Bovetto, L.; Barbar, R.; Sanchez, C. Multiscale characterization of individualized β-lactoglobulin microgels formed upon heat treatment under narrow pH range conditions. Langmuir 2009, 25, 7899–7909. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Munoz, J.A.; Solanilla, J.F.; Vaquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef]
- Ghani, S.; Barzegar, H.; Noshad, M.; Hojjati, M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int. J. Biol. Macromol. 2018, 112, 197–202. [Google Scholar] [CrossRef]
- Liu., Q.R.; Wang., W.; Qi., J.; Huang., Q.; Xiao, J. Oregano essential oil loaded soybean polysaccharide films: Effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll. 2019, 87, 165–172. [Google Scholar] [CrossRef]
- Arnaudov, L.N.; De, V.R.; Ippel, H.; van Mierlo, C.P. Multiple steps during the formation of beta-lactoglobulin fibrils. Biomacromolecules 2003, 4, 1614. [Google Scholar] [CrossRef]
- Uzun, S.; Kim, H.; Leal, C.; Padua, G.W. Ethanol-induced whey protein gels as carriers for lutein droplets. Food Hydrocoll. 2016, 61, 426–432. [Google Scholar] [CrossRef]
- Serfert, Y.; Lamprecht, C.; Tan, C.P.; Keppler, J.K.; Appel, E.; Rossier-Miranda, F.J.; Schroen, K.; Boom, R.M.; Gorb, S.; Selhuber-Unkel, C.; et al. Characterisation and use of β-lactoglobulin fibrils for microencapsulation of lipophilic ingredients and oxidative stability thereof. J. Food Eng. 2014, 143, 53–61. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Eco-Innovation in Reusing Food By-Products: Separation of Ovalbumin from Salted Egg White Using Aqueous Two-Phase System of PEG 1000/(NH4)2SO4. Polymers 2019, 11, 238. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Ma, Y.; Ngadi, M.O. Binding of curcumin to beta-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Huang, G.; Liu, J.; Slavin, M.; Yu, L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int. J. Biol. Macromol. 2016, 89, 499–506. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional Materials in Food Nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Barbosa-Canovas, G.V. Processing of soft Hispanic cheese (“queso fresco”) using thermo-sonicated milk: A study of physicochemical characteristics and storage life. J. Food Sci. 2010, 75, S548–S558. [Google Scholar] [CrossRef] [PubMed]







| Emulsions | β-Sheet (%) | Random Coils (%) | α-Helix (%) | β-Turn (%) |
|---|---|---|---|---|
| WPI/Gly | 28.62 | 21.76 | 18.51 | 31.11 |
| WPNFs/Gly | 46.52 | 17.17 | 13.97 | 22.34 |
| WPI-CA/Gly | 26.36 | 17.59 | 21.53 | 34.52 |
| WPNFs-CA/Gly | 42.04 | 16.79 | 15.69 | 23.48 |
| Films | Thickness (mm) | Transparency (%) | Moisture Content (%) |
|---|---|---|---|
| WPI/Gly | 0.184 ± 0.066b | 45.7 ± 1.3b | 44.2 ± 1.6a |
| WPNFs/Gly | 0.182 ± 0.034b | 49.2 ± 1.4a | 32.8 ± 0.9b |
| WPI-CA/Gly | 0.232 ± 0.045a | 41.5 ± 0.1c | 34.6 ± 1.1b |
| WPNFs-CA/Gly | 0.226 ± 0.038a | 49.7 ± 1.1a | 26.0 ± 1.2c |
| Films | Hardness (N) | Cohesiveness Area (B/A) | Chewiness (N/m) | Springiness (Mm) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | |
| Control | 23.08 ± 0.47e | 59.49 ± 0.12a | 0.65 ± 0.04a | -- | 15.48 ± 0.88b | -- | 0.91 ± 0.03a | -- |
| WPI/Gly | 19.70 ± 0.28f | 58.57 ± 0.24b | 0.54 ± 0.06b | -- | 14.13 ± 0.24b | -- | 0.81 ± 0.02a | -- |
| WPI-CA/Gly | 19.42 ± 0.14f | 58.18 ± 0.34b | 0.52 ± 0.01b | -- | 14.87 ± 0.27b | -- | 0.84 ± 0.03a | -- |
| WPNFs/Gly | 19.10 ± 0.63f | 52.37 ± 0.44c | 0.55 ± 0.04b | 0.42 ± 0.04c | 15.44 ± 0.37b | 27.94 ± 0.24a | 0.84 ± 0.11a | 0.89 ± 0.01a |
| WPNFs-CA/Gly | 18.40 ± 0.58f | 51.11 ± 0.32d | 0.53 ± 0.03b | 0.43 ± 0.04c | 15.94 ± 0.53b | 28.22 ± 0.36a | 0.91 ± 0.01a | 0.89 ± 0.10a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings 2019, 9, 583. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9090583
Wang Q, Yu H, Tian B, Jiang B, Xu J, Li D, Feng Z, Liu C. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings. 2019; 9(9):583. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9090583
Chicago/Turabian StyleWang, Qiannan, Hongliang Yu, Bo Tian, Bin Jiang, Jing Xu, Dongmei Li, Zhibiao Feng, and Chunhong Liu. 2019. "Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese" Coatings 9, no. 9: 583. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings9090583