Ceriporia lacerata Mycelium Culture Medium as a Novel Anti-Aging Microbial Material for Cosmeceutical Application
Abstract
:1. Introduction
2. Materials and Methods
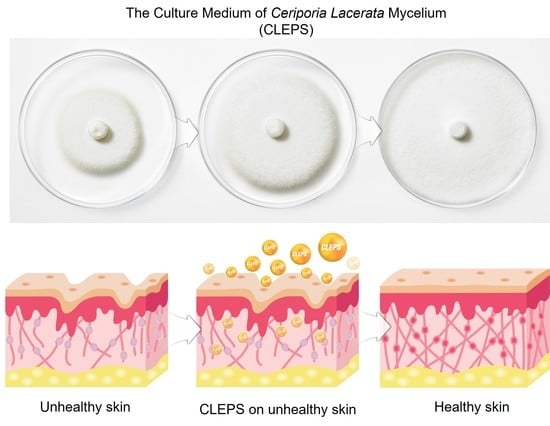
2.1. Ceriporia Lacerata Exo-Pharmaceutical Substance Preparation
2.2. Measurement of Antioxidant Activity
2.2.1. 2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Scavenging Activity Assay
2.2.2. 2,2-Azinobis-(3-ethylbenzothiazoline-sulfonic acid) (ABTS) Radical Scavenging Activity
2.3. Cell Viability Assay
2.4. Expression Level of Filaggrin
2.5. Melanogenesis Inhibition Test of B16 Melanoma Cells
2.6. Anti-Inflammation Assay of Nitric Oxide (NO)
2.7. Anti-Inflammation Assay of Inducible Nitric Oxide Synthase (iNOS), Cyclooxygenase-2 (COX2), and Tumor Necrosis Factor α (TNF α)
2.8. Synthesis of Collagen and Inhibition of Collagenase
2.9. In Vitro Wound Healing Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Measurement of Antioxidant Activity
3.2. Cell Viability Assay
3.3. Expression Level of Filaggrin
3.4. Melanogenesis Inhibition Test of B16 Melanoma Cells
3.5. Anti-Inflammation Assay of NO, iNOS, COX2, and TNFα
3.6. Synthesis of Collagen and Inhibition of Collagenase
3.7. In Vitro Wound Healing Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end-products |
| CLEPS | Ceriporia lacerata exo-pharmaceutical substance |
| EPS | exopolysaccharides |
| MMPs | Matrix metalloproteinases |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| LPS | lipopolysaccharide |
| α-LA | α-lipoic acid |
| iNOS | inducible nitric oxide synthase |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| ABTS | 2,2-azinobis-(3-ethylbenzothiazoline-sulfonic acid) |
| UV | Ultraviolet |
References
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of microbial products in cosmetic industry. Nat. Prod. Bioprospect. 2019, 9, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Chang, C.J.; Young, J.D. Antiaging effects of bioactive molecules isolated from plants and fungi. Med. Res. Rev. 2019, 39, 1515–1552. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function, and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Basenko, E.Y.; Philipp Benz, J.; Braus, G.H.; Caddick, M.X.; Csukai, M.; Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Dufossé, L.; Fouillaud, M.; Caro, Y. Fungi and fungal metabolites for the improvement of human and animal nutrition and health. J. Fungi 2021, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Barbosa, B.; Martins, B.A.P.; Guirlanda, C.A.F.; Moura, M. Use of the versatility of fungal metabolism to meet modern demands for healthy aging, functional foods, and sustainability. J. Fungi 2020, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Survase, S.A.; Singhal, R.S. Production of Schizophyllan using Schizophyllum commune NRCM. Bioresour. Technol. 2008, 99, 1036–1043. [Google Scholar] [CrossRef]
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily fluctuation of facial pore area, roughness, and redness among young Japanese women; beneficial effects of Galactomyces ferment filtrate containing antioxidative skin care formula. J. Clin. Med. 2021, 10, 2502. [Google Scholar] [CrossRef]
- Suhara, H.; Maekawa, N.; Kaneko, S.; Hattori, T.; Sakai, K.; Kondo, R. A new species, Ceriporia lacerata, isolated from white-rotted wood. Mycotaxon 2003, 86, 335–347. [Google Scholar]
- Lee, J.W.; Gwak, K.S.; Park, J.Y.; Park, M.J.; Choi, D.H.; Kwon, M.; Choi, I.G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 2007, 45, 485–491. [Google Scholar]
- Kim, J.E.; Kim, H.J.; Lee, S.P. Hyperglycemic effect of submerged culture solution of Ceriporia lacerata in streptozotocin-induced diabetic rats. Food Sci. Biotechnol. 2012, 21, 1685–1693. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.K.; Kim, J.E.; Lee, S.P.; Kim, B.C.; Jang, B.C. Crude solution of Ceriporia lacerata has a protective effect on dexamethasone-induced cytotoxicity in INS-1 cells via the modulation of PI3K/PKB activity. Int. J. Mol. Med. 2013, 32, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.J.; Kim, J.E.; Kim, J.H.; Park, Y.M.; Yoon, S.K.; Jang, B.C.; Lee, S.P.; Kim, B.C. Effect of submerged culture of Ceriporia lacerata mycelium on GLUT4 protein in db/db mouse. Korean J. Food Preserv. 2015, 22, 893–900. [Google Scholar] [CrossRef]
- Choi, J.W.; Shin, E.J.; Lee, S.J.; Kim, Y.H.; Kim, S.R.; Ji, Y.M.; Kim, N.Y.; An, C.H.; Lee, I.H.; Kim, Y.S. Effects of submerged culture of Ceriporia lacerata mycelium on high fat diet-induced diabetic mice with insulin resistance. J. Korean Soc. Food Sci. 2017, 46, 1419–1426. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Suh, H.J.; Kim, J.H.; Park, S.; Joo, Y.C.; Kim, J.S. Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae. J. Agric. Food Chem. 2010, 58, 11633–11638. [Google Scholar] [CrossRef]
- Goh, M.J.; Lee, H.K.; Cheng, L.; Kong, D.Y.; Yeon, J.H.; He, Q.Q.; Cho, J.C.; Na, Y.J. Depigmentation effect of kadsuralignan F on melan-a murine melanocytes and human skin equivalents. Int. J. Mol. Sci. 2013, 15, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef]
- Brown, S.J.; Mclean, W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, A.; Goto, M.; Mitsui, T.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidant Artemisia princeps Extract Enhances the Expression of Filaggrin and Loricrin via the AHR/OVOL1 Pathway. Int. J. Mol. Sci. 2017, 18, 1948. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto-Hachiya, A.; Tsuji, G.; Murai, M.; Yan, X.; Furue, M. Upregulation of FLG, LOR, and IVL Expression by Rhodiola crenulata Root Extract via Aryl Hydrocarbon Receptor: Differential Involvement of OVOL1. Int. J. Mol. Sci. 2018, 19, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Jeong, Y.; Park, J.-H.; Lee, C.H.; Yun, N.; Lee, D.S.; Nam, I.-J.; Kim, J.-D.; Yoon, K.D.; Son, M.; et al. Water-Soluble Extract from Actinidia arguta (Siebold & Zucc.) Planch. ex Miq. and Perilla frutescens (L.) Britton, ACTPER, Ameliorates a Dry Skin-Induced Itch in a Mice Model and Promotes Filaggrin Expression by Activating the AhR Signaling in HaCaT Cells. Nutrients 2019, 11, 1366. [Google Scholar]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015, 40, 786–793. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, D.; Zhang, Y.; Li, J.; Wu, Z.; Wang, Z. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int. J. Mol. Med. 2018, 41, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Blaut, M.; Braune, A.; Wunderlich, S.; Sauer, P.; Schneider, H.; Glatt, H. Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food Chem. Toxicol. 2006, 44, 1940–1947. [Google Scholar] [CrossRef]
- Gunawardena, D.; Bennett, L.; Shanmugam, K.; King, K.; Williams, R.; Zabaras, D.; Head, R.; Ooi, L.; Gyengesi, E.; Münch, G. Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-γ activated murine macrophages. Food Chem. 2014, 148, 92–96. [Google Scholar] [CrossRef]
- Sadowski, T.; Dietrich, S.; Müller, M.; Havlickova, B.; Schunck, M.; Proksch, E.; Müller, M.S.; Sedlacek, R. Matrix metalloproteinase-19 expression in normal and diseased skin: Dysregulation by epidermal proliferation. J. Investig. Dermatol. 2003, 121, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E. Exopolysaccharide Production and Antidiabetic Effect by Immersion Culture of Ceriporia lacerata. Ph.D. Thesis, Keimyung University Graduate School, Daegu, Korea, 2013. [Google Scholar]
- Shan, W.G.; Liang, D.E.; Ying, Y.M.; Zhan, Z.J. Two new tremulane sesquiterpenoids from Ceriporia lacerate, an endophytic fungus of Huperzia serrata. J. Chem. Res. 2012, 36, 365–366. [Google Scholar] [CrossRef]
- Ying, Y.M.; Shan, W.G.; Zhang, L.W.; Zhan, Z.J. Ceriponols A-K, tremulane sesquitepenes from Ceriporia lacerate HS-ZJUTC13A, a fungal endophyte of Huperzia serrata. Phytochemistry 2013, 95, 360–367. [Google Scholar] [CrossRef] [PubMed]







| Genes | Sequences | Accession No. | Amplicon Size, bp |
|---|---|---|---|
| β-actin (Human) | Forward 5’-GTCACCAACTGGGACGACATG-3 Reverse 5’-GCCGTCAGGCAGCTCGTAGC-3’ | NC_000019 | 640 |
| Filaggrin (Human) | Forward 5’-TGATGCAGTCTCCCTCTGTG-3 Reverse 5’-TGTTTCTCTTGGGCTCTTGG-3’ | NC_000001 | 610 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; An, C.; Hwang, S.D.; Kim, Y.S. Ceriporia lacerata Mycelium Culture Medium as a Novel Anti-Aging Microbial Material for Cosmeceutical Application. Cosmetics 2021, 8, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8040101
Kim J-H, An C, Hwang SD, Kim YS. Ceriporia lacerata Mycelium Culture Medium as a Novel Anti-Aging Microbial Material for Cosmeceutical Application. Cosmetics. 2021; 8(4):101. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8040101
Chicago/Turabian StyleKim, Jeong-Hwan, Changhun An, Seong Deok Hwang, and Yoon Soo Kim. 2021. "Ceriporia lacerata Mycelium Culture Medium as a Novel Anti-Aging Microbial Material for Cosmeceutical Application" Cosmetics 8, no. 4: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/cosmetics8040101