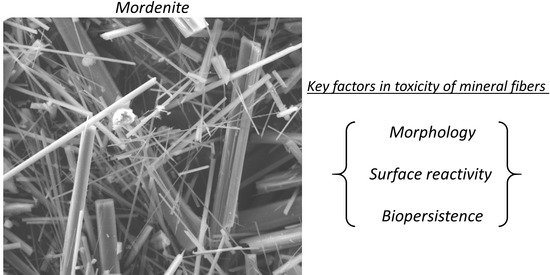
Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mordenite Sample
2.2. Electron Microscopy
2.3. Fiber Density and Aerodynamic Diameter
2.4. Determination of the Zeta Potential
2.5. Biodurability
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sisko, A.; Boffetta, P. Occupational Cancers; Springer Nature: London, UK, 2020; p. 640. [Google Scholar]
- IARC (International Agency for Research on Cancer). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monogr. Eval. Carcinog Risks Hum. C 2012, 100, 219–309. [Google Scholar]
- Gualtieri, A.F. Mineral fibre-based building materials and their health hazards. In Toxicity of Building Materials; Woodhead Publishing: Cambridge, UK, 2012; pp. 166–195. [Google Scholar]
- Carbone, M.; Kanodia, S.; Chao, A.; Miller, A.; Wali, A.; Weissman, D.; Adjei, A.; Baumann, F.; Boffetta, P.; Buck, B.; et al. Consensus Report of the 2015 Weinman International Conference on Mesothelioma. J. Thorac. Oncol. 2016, 11, 1246–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middendorf, P.; Zumwalde, R.; Castellan, R.; Harper, M.; Wallace, W.; Stayner, L.; Castranova, V.; Hearl, F.; Sullivan, P.; Wallace, W.; et al. Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research; NIOSH Current Intelligence Bulletin 62; National Institute for Occupational Safety and Health (NIOSH): Cincinnati, OH, USA, 2011; p. 174. [Google Scholar]
- Baumann, F.; Buck, B.J.; Metcalf, R.V.; McLaurin, B.T.; Merkler, D.J.; Carbone, M. The Presence of Asbestos in the Natural Environment is Likely Related to Mesothelioma in Young Individuals and Women from Southern Nevada. J. Thorac. Oncol. 2015, 10, 731–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, T.C.; Antao, V.C.; Bove, F.J. Vermiculite worker mortality: Estimated effects of occupational exposure to Libby amphibole. J. Occup. Environ. Med. 2010, 52, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Comba, P.; Gianfagna, A.; Paoletti, L. Pleural mesothelioma cases in Biancavilla are related to a new fluoro-edenite fibrous amphibole. Arch. Environ. Health 2003, 58, 229–232. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Harper, M.; Bailey, M.; Erskine, B.; Della Ventura, G.; Ardith, M.; Pasquali, L.; Tomaino, G.; Ray, R.; Mason, H.; et al. Characterization and assessment of the potential toxicity/pathogenicity of fibrous glaucophane. Environ. Res. 2019, 178, 108723. [Google Scholar] [CrossRef]
- Petriglieri, J.R.; Laporte-Magoni, C.; Salvioli-Mariani, E.; Tomatis, M.; Gazzano, E.; Turci, F.; Cavallo, A.; Fubini, B. Identification and Preliminary Toxicological Assessment of a Non-Regulated Mineral Fiber: Fibrous Antigorite from New Caledonia. Environ. Eng. Geosci. 2020, 26, 89–97. [Google Scholar] [CrossRef]
- Groppo, C.; Tomatis, M.; Turci, F.; Gazzano, E.; Ghigo, D.; Compagnoni, R.; Fubini, B. Potential toxicity of nonregulated asbestiform minerals: Balangeroite from the western Alps. Part 1: Identification and characterization. J. Toxicol. Environ. Health A 2005, 68, 1–19. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, M.; Giordani, M.; Arcangeli, P.; Valentini, L.; Boscardin, M.; Pacella, A.; Ballirano, P. Prismatic to asbestiform offretite from Northern Italy: Occurrence, morphology and crystal-chemistry of a new potentially hazardous zeolite. Minerals 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Zoboli, A.; Di Giuseppe, D.; Baraldi, C.; Gamberini, M.C.; Malferrari, D.; Urso, G.; Gualtieri, M.L.; Bailey, M.; Gualtieri, A.F. Characterisation of fibrous ferrierite in the rhyolitic tuffs at Lovelock, Nevada, USA. Mineral. Mag. 2019, 83, 577–586. [Google Scholar] [CrossRef]
- Carbone, M.; Emri, S.; Dogan, A.U.; Steele, I.; Tuncer, M.; Pass, H.I.; Baris, Y.I. A mesothelioma epidemic in Cappadocia: Scientific developments and unexpected social outcomes. Nat. Rev. Cancer 2007, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Gandolfi, N.B.; Passaglia, E.; Pollastri, S.; Mattioli, M.; Giordani, M.; Ottaviani, M.F.; Cangiotti, M.; Bloise, A.; Barca, D.; et al. Is fibrous ferrierite a potential health hazard? Characterization and comparison with fibrous erionite. Am. Mineral. 2018, 103, 1044–1055. [Google Scholar] [CrossRef]
- Tschernich, R.W. Zeolites of the World; Geoscience Press: Tucson, AZ, USA, 1992; p. 563. [Google Scholar]
- Stephenson, D.J.; Fairchild, C.I.; Buchan, R.M.; Dakins, M.E. A fiber characterization of the natural zeolite, mordenite: A potential inhalation health hazard. Aerosol Sci. Technol. 1999, 30, 467–476. [Google Scholar] [CrossRef]
- Passaglia, E. The Crystal Chemistry of Mordenites. Contrib. Mineral. Petrol. 1975, 50, 65–77. [Google Scholar] [CrossRef]
- Meier, W.M. The crystal structure of mordenite (ptilolite). Z. Krist. 1961, 115, 439–450. [Google Scholar] [CrossRef]
- How, D.C.L. On mordenite, a new mineral from the trap of Nova Scotia. J. Chem. Soc. 1984, 17, 100–104. [Google Scholar]
- Simoncic, P.; Armbruster, T. Peculiarity and defect structure of the natural and synthetic zeolite mordenite: A single-crystal X-ray study. Am. Mineral. 2004, 89, 421–431. [Google Scholar] [CrossRef]
- Martucci, A.; Sacerdoti, M.; Cruciani, G.; Dalconi, C. In situ time resolved synchrotron powder diffraction study of mordenite. Eur. J. Mineral. 2003, 15, 485–493. [Google Scholar] [CrossRef]
- Passaglia, E.; Sheppard, R.A. The crystal chemistry of zeolites. In Natural Zeolites: Occurrence, Properties Application; Bish, D.L., Ming, D.W., Eds.; Mineralogical Society of America and Geochemical Society: Washington, DC, USA, 2001; pp. 69–116. [Google Scholar]
- Zhang, L.; Xie, S.; Xin, W.; Li, X.; Liu, S.; Xu, L. Crystallization and morphology of mordenite zeolite influenced by various parameters in organic-free synthesis. Mater. Res. Bull. 2011, 46, 894–900. [Google Scholar] [CrossRef]
- Passaglia, E.; Artioli, G.; Gualtieri, A.; Carnevali, R. Diagenetic mordenite from Ponza, Italy. Eur. J. Mineral. 1995, 7, 429–438. [Google Scholar] [CrossRef]
- Godelitsas, A.; Gamaletsos, P.; Roussos-Kotsis, M. Mordenite-bearing tuffs from Prassa quarry, Kimolos Island, Greece. Eur. J. Mineral. 2010, 22, 797–811. [Google Scholar] [CrossRef]
- Negishi, T. Mordenite in the tuffs of the Shirasawa District, Miyagl Prefecture. J. Jpn. Assoc. Mineral. Petrol. Econ. Geol. 1972, 67, 29–34. [Google Scholar] [CrossRef] [Green Version]
- IARC (International Agency for Research on Cancer). Silica, some silicates, coal dust and para-aramid fibrils. IARC Monogr. Eval. Carcinog. Risks Hum. C 1997, 68, 307–337. [Google Scholar]
- IARC Monographs on the IDENTIFICATION of CARCINOGENIC Hazards to Humans. Available online: https://monographs.iarc.fr/wp-content/uploads/2019/07/Preamble-2019.pdf (accessed on 2 August 2020).
- Suzuki, Y. Carcinogenic and fibrogenic effects of zeolites: Preliminary observations. Environ. Res. 1982, 27, 433–445. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kohyama, N. Malignant mesothelioma induced by asbestos and zeolite in the mouse peritoneal cavity. Environ. Res. 1984, 35, 277–292. [Google Scholar] [CrossRef]
- Tátrai, E.; Bácsy, E.; Kárpáti, J.; Ungváry, G. On the examination of the pulmonary toxicity of mordenite in rats. Pol. J. Occup. Med. Environ. Health 1992, 5, 237–243. [Google Scholar]
- Adamis, Z.; Tátrai, E.; Honma, K.; Six, É.; Ungváry, G. In Vitro and in Vivo Tests for Determination of the Pathogenicity of Quartz, Diatomaceous Earth, Mordenite and Clinoptilolite. Ann. Occup. Hyg. 2000, 44, 67–74. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Towards a quantitative model to predict the toxicity/pathogenicity potential of mineral fibers. Toxicol. Appl. Pharm. 2018, 361, 89–98. [Google Scholar] [CrossRef]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateu-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA-Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [Green Version]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giuseppe, D.; Zoboli, A.; Vigliaturo, R.; Gieré, R.; Bonasoni, M.P.; Sala, O.; Gualtieri, A.F. Mineral fibres and asbestos bodies in human lung tissue: A case study. Minerals 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. BBA-Gen. Subj. 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.J.; Liang, M.L.; Tóth, I.; Monteiro, M.J.; Michin, R.F. Molecular interaction of poly (acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano 2012, 6, 8962–8969. [Google Scholar] [CrossRef]
- Harris, R.L.; Timbrell, V. Relation of alveolar deposition to the diameter and length of glass fibres. In Proceedings of the Inhaled Particles IV International Symposium Organized by the British Occupational Hygiene Society, Edinburgh, UK, 22–26 September 1977; Pergamon Press: Oxford, UK, 1977; p. 411. [Google Scholar]
- Yeh, H.C.; Phalen, R.F.; Raabe, O.G. Factors influencing the deposition of inhaled particles. Environ. Health Perspect. 1976, 15, 147–156. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health Part B 2011, 14, 40–75. [Google Scholar] [CrossRef] [Green Version]
- Bonneau, L.; Suquet, H.; Malard, C.; Pezerat, H. Studies on surface properties of asbestos: I. active sites on surface of chrysotile and amphiboles. Environ. Res. 1986, 41, 251–267. [Google Scholar] [CrossRef]
- Turci, F.; Tomatis, M.; Lesci, I.G.; Roveri, N.; Fubini, B. The iron-related molecular toxicity mechanism of synthetic asbestos nanofibres: A model study for high-aspect-ratio nanoparticles. Chem. Eur. J. 2011, 17, 350–358. [Google Scholar] [CrossRef]
- Hardy, J.A.; Aust, A.E. Iron in asbestos chemistry and carcinogenicity. Chem. Rev. 1995, 95, 97–118. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Andreozzi, G.B.; Tomatis, M.; Turci, F. Iron from a geochemical viewpoint. Understanding toxicity/pathogenicity mechanisms in iron-bearing minerals with a special attention to mineral fibers. Free Radic. Bio. Med. 2019, 133, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Gualtieri, A.F.; Ignatyev, K.; Strafella, E.; Pugnaloni, A.; Croce, A. Stability of mineral fibres in contact with human cell cultures. An in situ μXANES, μXRD and XRF iron mapping study. Chemosphere 2016, 164, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Gualtieri, A.F.; Gualtieri, M.L.; Hanuskova, M.; Cavallo, A.; Gaudino, G. The zeta potential of mineral fibres. J. Hazard. Mater. 2014, 276, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Pollastri, S.; Gandolfi, N.B.; Gualtieri, M.L. In vitro acellular dissolution of mineral fibres: A comparative study. Sci. Rep. 2018, 8, 7071. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Chevalier, J.; Smith, P. Comparison of Calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal. Toxicol. 2005, 17, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Sukheswala, R.N.; Avasia, R.K.; Gangopadhyay, M. Zeolites and associated secondary minerals in the Deccan Traps of Western India. Mineral. Mag. 1974, 39, 658–671. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Mental Health. ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 14 March 2020).
- Gualtieri, A.F.; Mossman, B.T.; Roggli, V.L. Towards a general model for predicting the toxicity and pathogenicity of minerals fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 501–526. [Google Scholar]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- French, C.A. Respiratory tract. In Cytology: Diagnostic Principles and Clinical Correlates; Cibas, E.S., Ducatman, B.S., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2009; pp. 65–103. [Google Scholar]
- Gonda, I.; Abd El Khalik, A.F. On the calculation of aerodynamic diameters of fibers. Aerosol. Sci. Technol. 1985, 4, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, D. The ζ-potential of glass surface in contact with aqueous solutions. J. Colloid Interface Sci. 2000, 226, 328–339. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Stern, O. The theory of the electrolytic double-layer. Z. Elektrochem. 1924, 30, 508–516. [Google Scholar]
- Gualtieri, A.F.; Lusvardi, G.; Zoboli, A.; Di Giuseppe, D.; Gualtieri, M.L. Biodurability and release of metals during the dissolution of chrysotile, crocidolite and fibrous erionite. Environ. Res. 2019, 171, 550–557. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Determination of Airborne Fibre Number Concentrations. Available online: http://apps.who.int/iris/bitstream/10665/41904/1/9241544961.pdf (accessed on 4 August 2020).
- Mattioli, M.; Giordani, M.; Dogan, M.; Cangiotti, M.; Avella, G.; Giorgi, R.; Dogan, U.A.; Ottaviani, M.F. Morpho-chemical characterization and surface properties of carcinogenic zeolite fibers. J. Hazard. Mater. 2016, 306, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.; Allegrina, M.; Rinaudo, C.; Gaudino, G.; Yang, H.; Carbone, M. Numerous iron-rich particles lie on the surface of erionite fibers from Rome (Oregon, USA) and Karlik (Cappadocia, Turkey). Microsc. Microanal. 2015, 21, 1341–1347. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Gandolfi, N.B.; Pollastri, S.; Pollok, K.; Langenhorst, F. Where is iron in erionite? A multidisciplinary study on fibrous erionite-Na from Jersey (Nevada, USA). Sci. Rep. 2016, 6, 37981. [Google Scholar] [CrossRef]
- Ballirano, P.; Andreozzi, G.B.; Dogan, M.; Dogan, A.U. Crystal structure and iron topochemistry of erionite-K from Rome, Oregon, USA. Am. Mineral. 2009, 94, 1262–1270. [Google Scholar] [CrossRef]
- Matassa, R.; Familiari, G.; Relucenti, M.; Battaglione, E.; Downing, C.; Pacella, A.; Cametti, G.; Ballirano, P. A deep look into erionite fibres: An electron microscopy investigation of their self-assembly. Sci. Rep. 2015, 5, 16757. [Google Scholar] [CrossRef] [Green Version]
- Hartman, R.L.; Fogler, H.S. Understanding the dissolution of zeolites. Langmuir 2007, 23, 5477–5484. [Google Scholar] [CrossRef]
- Urano, N.; Yano, E.; Evans, P.H. Reactive oxygen metabolites produced by the carcinogenic fibrous mineral erionite. Environ. Res. 1991, 54, 74–81. [Google Scholar] [CrossRef]
- Pacella, A.; Cremisini, C.; Nardi, E.; Montereali, M.R.; Pettiti, I.; Giordani, M.; Mattioli, M.; Ballirano, P. Different erionite species bind iron into the structure: A potential explanation for fibrous erionite toxicity. Minerals 2018, 8, 36. [Google Scholar] [CrossRef] [Green Version]






| Percentiles * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Min | 5th | 25th | 50th | 75th | 95th | Max | σ | |
| L (µm) | 2.09 | 3.52 | 11.2 | 20.8 | 36.7 | 56.1 | 118 | 20.5 |
| W (µm) | 0.11 | 0.18 | 0.61 | 0.95 | 1.24 | 1.80 | 3.34 | 0.58 |
| wt% | Mordenite Poona (India) | Erionite Nevada (USA) | Erionite Karain (Turkey) |
|---|---|---|---|
| SiO2 | 68.82(1.57) | 58.47(0.35) | 56.66(0.32) |
| Al2O3 | 10.35(0.92) | 14.30(0.21) | 13.63(0.27) |
| Fe2O3 | 0.20(0.03) | 0.13(0.05) | 0.07(0.04) |
| MgO | 0.08(0.01) | 0.48(0.12) | 0.02(0.10) |
| CaO | 2.95(0.59) | 3.35(0.23) | 1.85(0.10) |
| Na2O | 3.17(0.98) | 0.80(0.10) | 2.48(0.16) |
| K2O | 0.21(0.09) | 4.17(0.29) | 5.49(0.45) |
| H2O | 14.0 * | 18.46(0.45) | 19.60(0.42) |
| Tot | 99.98(1.84) | 81.54 | 80.40 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuseppe, D. Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity. Crystals 2020, 10, 769. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090769
Di Giuseppe D. Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity. Crystals. 2020; 10(9):769. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090769
Chicago/Turabian StyleDi Giuseppe, Dario. 2020. "Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity" Crystals 10, no. 9: 769. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090769