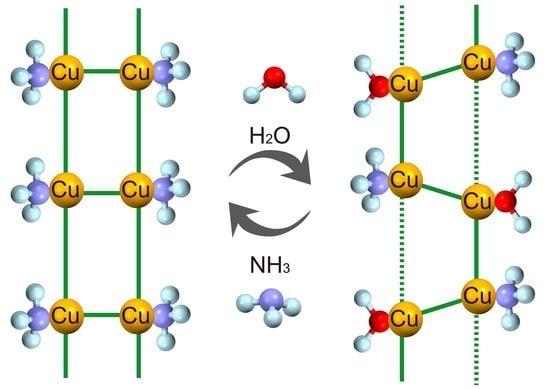
Gas-Dependent Reversible Structural and Magnetic Transformation between Two Ladder Compounds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Magnetic Anomaly of Compound 1
3.2. Transformation from Compound 1 to 2
3.3. Transformation from Compound 2 to 1
3.4. Reversible Transformation between Compounds 1 and 2
3.5. Stability of Compounds 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Dagotto, E.; Riera, J.; Scalapino, D. Superconductivity in ladders and coupled planes. Phys. Rev. B 1992, 45, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.M.; Gopalan, S.; Sigrist, M. Superconductivity, Spin Gaps and Luttinger Liquids in a Class of Cuprates. Europhys. Lett. 1993, 23, 445–449. [Google Scholar] [CrossRef]
- Dagotto, E.; Rice, T.M. Surprises on the Way from One- to Two-Dimensional Quantum Magnets: The Ladder Materials. Science 1996, 271, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Azuma, M.; Hiroi, Z.; Takano, M.; Ishida, K.; Kitaoka, Y. Observation of a Spin Gap in SrCu2O3 Comprising Spin-½ Quasi-1D Two-Leg Ladders. Phys. Rev. Lett. 1994, 73, 3463–3466. [Google Scholar] [CrossRef]
- Ishida, K.; Kitaoka, Y.; Asayama, K.; Azuma, M.; Hiroi, Z.; Takano, M. Spin Gap Behavior in Ladder-Type of Quasi-One-Dimensional Spin (S = 1/2) System SrCu2O3. J. Phys. Soc. Jpn. 1994, 63, 3222–3225. [Google Scholar] [CrossRef]
- Azuma, M.; Yoshida, H.; Saito, T.; Yamada, T.; Takano, M. Pressure-Induced Buckling of Spin Ladder in SrCu2O3. J. Am. Chem. Soc. 2004, 126, 8244–8246. [Google Scholar] [CrossRef]
- Kojima, K.; Keren, A.; Luke, G.M.; Nachumi, B.; Wu, W.D.; Uemura, Y.J.; Azuma, M.; Takano, M. Magnetic Behavior of the 2-Leg and 3-Leg Spin Ladder Cuprates Srn−1Cun+1O2n. Phys. Rev. Lett. 1995, 74, 2812–2815. [Google Scholar] [CrossRef]
- Rovira, C. Molecular Spin Ladders. Chem. Eur. J. 2000, 6, 1723–1729. [Google Scholar] [CrossRef]
- Nishihara, S.; Akutagawa, T.; Hasegawa, T.; Nakamura, T. Formation of a molecular spin ladder induced by a supramolecular cation structure. Chem. Commun. 2002, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, S.; Akutagawa, T.; Hasegawa, T.; Fujiyama, S.; Nakamura, T.; Nakamura, T. Two Polymorphs of (Anilinium)(18-Crown-6)[Ni(dmit)2]: Structure and Magnetic Properties. J. Solid State Chem. 2002, 168, 661–667. [Google Scholar] [CrossRef]
- Nishihara, S.; Akutagawa, T.; Hasegawa, T.; Nakamura, T.; Fujiyama, S.; Nakamura, T. Magnetic and 1H-NMR spectroscopic studies of [Ph(NH3)](18-crown-6) [Ni(dmit)2] having molecular spin ladder structure. Synth. Met. 2003, 137, 1279–1280. [Google Scholar] [CrossRef]
- Ichihashi, K.; Konno, D.; Date, T.; Nishimura, T.; Maryunina, K.Y.; Inoue, K.; Nakaya, T.; Toyoda, K.; Tatewaki, Y.; Akutagawa, T.; et al. Optimizing Lithium Ion Conduction through Crown Ether-Based Cylindrical Channels in [Ni(dmit)2]− Salts. Chem. Mater. 2018, 30, 7130–7137. [Google Scholar] [CrossRef]
- Ichihashi, K.; Konno, D.; Maryunina, K.Y.; Inoue, K.; Toyoda, K.; Kawaguchi, S.; Kubota, Y.; Tatewaki, Y.; Akutagawa, T.; Nakamura, T.; et al. Selective Ion Exchange in Supramolecular Channels in the Crystalline State. Angew. Chem. Int. Ed. 2019, 131, 4213–4216. [Google Scholar] [CrossRef]
- Zhang, X.; Nishihara, S.; Nakano, Y.; Yoshida, E.; Kato, C.; Ren, X.-M.; Maryunina, K.Y.; Inoue, K. A magnetically isolated cuprate spin-ladder system: Synthesis, structures, and magnetic properties. Dalton Trans. 2014, 43, 12974–12981. [Google Scholar] [CrossRef] [Green Version]
- Johnston, D.C.; Troyer, M.; Miyahara, S.; Lidsky, D.; Ueda, K.; Azuma, M.; Hiroi, Z.; Takano, M.; Isobe, M.; Ueda, Y.; et al. Magnetic Susceptibilities of Spin-1/2 Antiferromagnetic Heisenberg Ladders and Applications to Ladder Oxide Compounds. arXiv 2000, arXiv:con-mat/0001147. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Kobori, S.; Matsui, K.; Kuwahara, H.; Goto, T.; Zhang, X.; Nakano, Y.; Nishihara, S.; Inoue, K.; Sasaki, T. NMR study on the quasi one-dimensional quantum spin magnet with ladder structure. Hyperfine Interact. 2016, 237, 116. [Google Scholar] [CrossRef]
- Zhang, X.; Nishihara, S.; Nakano, Y.; Maryunina, K.Y.; Inoue, K. A Cuprate Spin Ladder Linked by a Pyridyl Ligand. Chem. Lett. 2014, 43, 1713–1715. [Google Scholar] [CrossRef] [Green Version]
- Azuma, M.; Fujishiro, Y.; Takano, M.; Nohara, M.; Takagi, H. Switching of the gapped singlet spin-liquid state to an antiferromagnetically ordered state in Sr(Cu1−xZnx)2O3. Phys. Rev. B 1997, 55, R8658–R8661. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yasuoka, H.; Fujishiro, Y.; Azuma, M.; Takano, M. NMR Study of Zn Doping Effect in Spin Ladder System SrCu2O3. Phys. Rev. Lett. 1998, 80, 604–607. [Google Scholar] [CrossRef]
- Ohsugi, S.; Tokunaga, Y.; Ishida, K.; Kitaoka, Y.; Azuma, M.; Fujishiro, Y.; Takano, M. Impurity-induced staggered polarization and antiferromagnetic order in spin-1/2 Heisenberg two-leg ladder compound SrCu2O3: Extensive Cu NMR and NQR studies. Phys. Rev. B 1999, 60, 4181–4190. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manabe, J.; Nishida, K.; Zhang, X.; Nakano, Y.; Fujibayashi, M.; Cosquer, G.; Inoue, K.; Shimono, S.; Ishibashi, H.; Kubota, Y.; et al. Gas-Dependent Reversible Structural and Magnetic Transformation between Two Ladder Compounds. Crystals 2020, 10, 841. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090841
Manabe J, Nishida K, Zhang X, Nakano Y, Fujibayashi M, Cosquer G, Inoue K, Shimono S, Ishibashi H, Kubota Y, et al. Gas-Dependent Reversible Structural and Magnetic Transformation between Two Ladder Compounds. Crystals. 2020; 10(9):841. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090841
Chicago/Turabian StyleManabe, Jun, Kazuki Nishida, Xiao Zhang, Yuki Nakano, Masaru Fujibayashi, Goulven Cosquer, Katsuya Inoue, Seiya Shimono, Hiroki Ishibashi, Yoshiki Kubota, and et al. 2020. "Gas-Dependent Reversible Structural and Magnetic Transformation between Two Ladder Compounds" Crystals 10, no. 9: 841. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090841