Growth Peculiarities and Properties of KR3F10 (R = Y, Tb) Single Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Equipment Design
2.2. Initial Chemical Reagents and Growth Parameters of Growth Process
2.3. X-ray Diffraction (XRD) Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Single-Crystal X-ray Diffraction Study of KR3F10
2.6. Optical Properties
2.7. The Thermal Conductivity Measurements
2.8. The Electrical Conductivity Measurements
3. Results and Discussion
3.1. Growth Process Results and Crystals Characterization
3.2. Crystal Structure Refinement
3.3. Optical Properties of KR3F10 Crystals
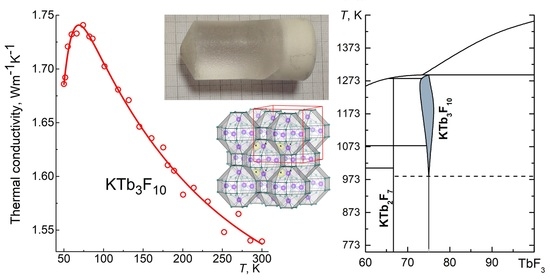
3.4. Thermal Conductivity Measurements
3.5. Ionic Conductivity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Denker, B.; Shklovsky, E. Handbook of Solid-State Lasers: Materials, Systems and Applications; Denker, B., Shklovsky, E., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Elsevier: Amsterdam, The Netherlands, 2013; p. 688. [Google Scholar]
- Kaminskii, A.A. Laser Crystals: Their Physics and Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1990; p. 456. [Google Scholar] [CrossRef]
- Scholle, K.; Lamrini, S.; Koopmann, P.; Fuhrberg, P. 2 μm laser sources and their possible applications. In Frontiers in Guided Wave Optics and Optoelectronics; Pal, B., Ed.; InTech: London, UK, 2010; pp. 471–500. [Google Scholar] [CrossRef] [Green Version]
- Abdulsabirov, R.Y.; Dubiniski, M.A.; Kazakov, N.M.; Silkin, N.I.; Yagudin, S.I. New Fluoride Laser Host. Sov. Phys. Crystallogr. 1987, 32, 559–564. [Google Scholar]
- Veronesi, S.; Parisi, D.; Marchetti, F.; Tonelli., M. Effect of Ce co-doping on KYF: Pr crystals. J. Phys. Chem. Solids 2010, 71, 913–917. [Google Scholar] [CrossRef] [Green Version]
- Serna-Gallén, P.; Beltrán-Mir, H.; Cordoncillo, E. Tuning the optical and photoluminescence properties of high efficient Eu3+-doped KY3F10 phosphors by different synthetic approaches. Opt. Laser Technol. 2021, 136, 106734. [Google Scholar] [CrossRef]
- Tigreat, P.Y.; Doualan, J.L.; Budasca, C.; Moncorge, R. Energy transfer processes in (Yb3+, Dy3+) and (Tm3+, Dy3+) codoped LiYF4 and KY3F10 single crystals. J. Lumin. 2001, 94–95, 23–27. [Google Scholar] [CrossRef]
- Braud, A.; Tigreat, P.Y.; Doualan, J.L.; Moncorgé, R. Spectroscopy and cw operation of a 1.85 μm Tm:KY3F10 laser. Appl. Phys. B 2001, 72, 909–912. [Google Scholar] [CrossRef]
- Bigotta, S.; Tonelli, M.; Cavalli, E.; Belletti, A. Optical spectra of Dy3+ in KY3F10 and LiLuF4 crystalline fibers. J. Lumin. 2010, 130, 13–17. [Google Scholar] [CrossRef]
- Loiko, P.; Doualan, J.-L.; Guillemot, L.; Moncorgé, R.; Starecki, F.; Benayad, A.; Dunina, E.; Kornienko, A.; Fomicheva, L.; Braud, A.; et al. Emission properties of Tm3+-doped CaF2, KY3F10, LiYF4, LiLuF4 and BaY2F8 crystals at 1.5 μm and 2.3 μm. J. Lumin. 2020, 225, 117279. [Google Scholar] [CrossRef]
- Kim, K.J.; Jouini, A.; Yoshikawa, A.; Simura, R.; Boulon, G.; Fukuda, T. Growth and optical properties of Pr, Yb-codoped KY3F10 fluoride single crystals for up-conversion visible luminescence. J. Cryst. Growth 2007, 299, 171–177. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Kamada, K.; Martin, N.; Aoki, K.; Sato, H.; Pejchal, J.; Fukuda, T. Growth and luminescent properties of Pr:KY3F10 single crystal. J. Cryst. Growth 2005, 285, 445–449. [Google Scholar] [CrossRef]
- Chen, M.; Loiko, P.; Serres, J.M.; Veronesi, S.; Tonelli, M.; Aguiló, M.; Díaz, F.; Choi, S.Y.; Bae, J.E.; Rotermund, F.; et al. Fluorite-type Tm3+:KY3F10: A promising crystal for watt-level lasers at ∼1.9μm. J. Alloy. Compd. 2020, 813, 152176. [Google Scholar] [CrossRef]
- Diaf, M.; Braud, A.; Labb, C.; Doualan, J.L.; Girard, S.; Margerie, J.; Moncorg, R.; Thuau, M. Synthesis and spectroscopic studies of Tm3+-doped KY3F10 single crystals. Can. J. Phys. 2000, 77, 693–697. [Google Scholar] [CrossRef]
- Guillemot, L.; Loiko, P.; Soulard, R.; Braud, A.; Doualan, J.-L.; Hideur, A.; Camy, P. Close look on cubic Tm:KY3F10 crystal for highly efficient lasing on the 3H4 → 3H5 transition. Opt. Express 2020, 28, 3451–3463. [Google Scholar] [CrossRef] [PubMed]
- Vasyliev, V.; Villora, E.G.; Nakamura, M.; Sugahara, Y.; Shimamura, K. UV-visible Faraday rotators based on rare-earth fluoride single crystals: LiREF4 (RE = Tb, Dy, Ho, Er and Yb), PrF3 and CeF3. Opt. Express 2012, 20, 14460. [Google Scholar] [CrossRef] [PubMed]
- Vojna, D.; Slezák, O.; Lucianetti, A.; Mocek, T. Verdet Constant of Magneto-Active Materials Developed for High-Power Faraday Devices. Appl. Sci. 2019, 9, 3160. [Google Scholar] [CrossRef] [Green Version]
- Valiev, U.V.; Karimov, D.N.; Burdick, G.W.; Rakhimov, R.; Pelenovich, V.O.; Fu, D. Growth and magnetooptical properties of anisotropic TbF3 single crystals. J. Appl. Phys. 2017, 121, 243105. [Google Scholar] [CrossRef] [Green Version]
- Zelmon, D.E.; Erdman, E.C.; Stevens, K.T.; Foundos, G.; Kim, J.R.; Brady, A. Optical properties of lithium terbium fluoride and implications for performance in high power lasers. Appl. Opt. 2016, 55, 834–837. [Google Scholar] [CrossRef]
- Pues, P.; Baur, F.; Schwung, S.; Rytz, D.; Pottgen, R.; Paulsen, C.; Janka, O.; Rendenbach, B.; Johrendt, D.; Jüstel, T. Temperature and time-dependent luminescence of single crystals of KTb3F10. J. Luminescence V 2020, 227, 117523. [Google Scholar] [CrossRef]
- Weber, M.J.; Morgret, R.; Leung, S.Y. Magneto-optical properties of KTb3F10 and LiTbF4 crystals. J. Appl. Phys. 1978, 49, 3464–3469. [Google Scholar] [CrossRef]
- Potassium Terbium Fluoride Crystal Growth Development for Faraday Rotator DISCS Fabrication, 6 July 1978–6 February 1979; Technical Report; Defensive Systems Div, Sanders Associates, Inc.: Nashua, NH, USA, May 1979. [CrossRef] [Green Version]
- Stevens, K.T.; Schlichting, W.; Foundos, G.; Payne, A.; Rogers, E. Promising materials for high power laser isolators: Growth of large single-crystals for faraday rotator and isolator applications. Laser Tech. J. 2016, 13, 18–21. [Google Scholar] [CrossRef]
- Schlichting, W.; Stevens, K.; Foundos, G.; Payne, A. Commercializing potassium terbium fluoride, KTF (KTb3F10) faraday crystals for high laser power optical isolator applications. Proc. SPIE 10448 Optifab. 2017, 10448, 104481N. [Google Scholar] [CrossRef]
- Karimov, D.N.; Buchinskaya, I.I. Growing KR3F10 (R = Tb–Er) crystals by the vertical directional crystallization method. I. Optimization of the melt composition for growing KTb3F10 and correction of the phase diagram of the KF–TbF3 system. Crystallogr. Rep. 2021, 66. in press. (in Russian) [Google Scholar] [CrossRef]
- Chamberlain, S.L.; Corruccini, L.R. Magnetic ordering in rare-earth fluorides with KY3F10 structure and axial moments. Phys. Rev. B 2005, 71, 024434-1-7. [Google Scholar] [CrossRef]
- Grzechnik, A.; Nuss, J.; Friese, K.; Gesland, J.-Y.; Jansen, M. Refinement of the crystal structure of potassium triyttrium decafluoride, KY3F10. Z. Kristallogr. NCS 2002, 217, 460. [Google Scholar] [CrossRef]
- Friese, K.; Krüger, H.; Kahlenberg, V.; Balić-Zunić, T.; Emerich, H.; Gesland, J.-Y.; Grzechnik, A. Study of the temperature dependence of the structure of KY3F10. J. Phys. Condens. Matter 2006, 18, 2677–2687. [Google Scholar] [CrossRef]
- Chai, B.; Lefaucheur, J.; Pham, A.-T.; Loutts, G.B.; Nicholls, J.F. Growth of high-quality single crystals of KYF4 by TSSG method. In Proceedings of the SPIE 1863, Growth, Characterization, and Applications of Laser Host and Nonlinear Crystals II, Los Angeles, CA, USA, 21 July 1993; pp. 131–135. [Google Scholar] [CrossRef]
- Zhangyang, K.; Christian, R.; Maogang, H.; Chartrand, P. Thermodynamic evaluation and optimization of the (KF+YF3), (KCl+YCl3) and (YF3+YbF3) binary systems. J. Chem. Thermodyn. 2016, 98, 242–253. [Google Scholar] [CrossRef]
- Krivandina, E.A. Preparation of single crystals of multicomponent fluoride materials with the fluorite type structure. Butll. Soc. Cat. Sien. 1991, 12, 393–412. [Google Scholar]
- Fedorov, P.P.; Osiko, V.V. Crystal Growth of Fluorides. In Bulk Crystal Growth of Electronic, Optical and Optoelectronic Materials; Capper, P., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 339–355. [Google Scholar] [CrossRef]
- Baldochi, S.L.; Ranieri, I.M. A Short Review on Fluoride Laser Crystals Grown by Czochralski Method at IPEN. Acta Phys. Pol. A 2013, 124, 286–294. [Google Scholar] [CrossRef]
- Baldochi, S.L.; Morato, S.P. Fluoride Bulk Crystals Growth. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 3200–3205. [Google Scholar]
- Fukuda, T.; Rudolph, P.; Uda, S. Fiber Crystal Growth from the Melt; Fukuda, T., Rudolph, P., Uda, S., Eds.; Series: Advances in Materials Research; Springer: Berlin/Heidelberg, Germany, 2004; p. 281. [Google Scholar] [CrossRef]
- Shu, J.; Damiano, E.; Sottile, A.; Zhang, Z.; Tonelli, M. Growth by the μ-PD Method and Visible Laser Operation of a Single-Crystal Fiber of Pr3+:KY3F10. Crystals 2017, 7, 200. [Google Scholar] [CrossRef] [Green Version]
- Deshko, V.I.; Zhmurova, Z.I.; Kalenichenko, S.G.; Karvatsky, A.Ya.; Krivandina, E.A.; Lebedeva, T.V.; Semenkov, Yu.S.; Sobolev, B.P. Investigation of temperature fields in a two-zone apparatus for growing fluoride crystals by the Stockbarger method. Crystallogr. Rep. 1994, 39, 485–495. [Google Scholar]
- Sobolev, B.P.; Stanishevskij, E.J.; Semenkov, J.V.; Kisel’kov, M.P.; Zubova, E.N.; Zhmurova, Z.I.; Krivandina, E.A. Device for Crystal Growing in Furnace with Two-Zone Electric Heating. Patent RU 2038356C1, 27 June 1995. [Google Scholar]
- Karimov, D.N.; Dymshits, Yu.M.; Markina, S.A.; Ivanovskaya, N.A.; Krivandina, E.A.; Sobolev, B.P. Crystal growing unit heater. Patent RU 131550U1, 20 November 2013. Bull. No. 23. [Google Scholar]
- Sobolev, B.P.; Lomova, V.I.; Karimov, D.N. Equipment for the Growing Crystals. Patent RU 120658U1, 27 November 2012. Bull. No. 27. [Google Scholar]
- Karimov, D.N.; Dymshits, Yu.M.; Zubova, E.A.; Samsonova, N.A.; Sorokin, N.I.; Sobolev, B.P. Crucible for the Crystals Growth. Patent RU 135321U1, 10 December 2013. Bull. No. 34. [Google Scholar]
- Blistanov, A.A.; Chernov, S.P.; Karimov, D.N.; Ouvarova, T.V. Peculiarities of the growth of disordered Na,R-fluorite (R = Y, Ce–Lu) single crystals. J. Cryst. Growth 2002, 237–239, 899–903. [Google Scholar] [CrossRef]
- Karimov, D.N.; Sobolev, B.P.; Ivanov, I.A.; Kanorsky, S.I.; Masalov, A.V. Growth and magneto-optical properties of Na0.37Tb0.63F2.26 cubic single crystal. Crystallogr. Rep. 2014, 59, 718–723. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Karimov, D.N.; Volchkov, I.S.; Grigor’ev, Y.V.; Sobolev, B.P. Fluorine-ionic conductivity of superionic conductor crystals Na0.37Tb0.63F2.26. Crystallogr. Rep. 2019, 64, 626–630. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction CrysAlis PRO Software System; Version 1.171.41.95a; Rigaku Corporation: Wroclaw, Poland, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.A.; Sidorov, A.A.; Kul’chenkov, E.A.; Аnishchenko, A.M.; Аvetisov, I.S.; Sorokin, N.I.; Fedorov, P.P. Thermal conductivity and expansion of PbF2 single crystal. Ionics 2017, 23, 233–239. [Google Scholar] [CrossRef]
- Opalovskii, A.A.; Fedotova, T.D. Metal Hydrogen Fluorides. Russ. Chem. Rev. 1970, 39, 1003–1016. [Google Scholar] [CrossRef]
- Mikou, A.; Laval, J.P.; Frit, B. Etude de l’ordre oxygene-fluor dans l’oxyfluorure PbZr3F6O4, isotype de KY3F10. Rev. Chim. Miner. 1985, 22, 115–124. [Google Scholar]
- Podberezskaya, N.V.; Potapova, O.G.; Borisov, S.V.; Gatilov, Y.V. Crystal structure of KTb3F10 cubic packing of the [Tb6F32]14- polyanions. J. Struct. Chem. 1976, 17, 815–817. [Google Scholar] [CrossRef]
- Ichikawa, R.U.; Linhares, H.M.S.M.D.; Peral, I.; Baldochi, S.L.; Ranieri, I.M.; Turrillas, X.; Martinez, L.G. Insights into the Local Structure of Tb-Doped KY3F10 Nanoparticles from Synchrotron X-ray Diffraction. ACS Omega 2017, 2, 5128–5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblanc, M.; Maisonneuve, V.; Tressaud, A. Crystal chemistry and selected physical properties of inorganic fluorides and oxide-fluorides. Chem. Rev. 2015, 115, 1191–1254. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Greis, O.; Strähle, J. A new structural principle in anion-excess fluorite-related superlattices. Acta Crystallogr. Sect. A 1980, 36, 889–890. [Google Scholar] [CrossRef]
- Golubev, A.M.; Garashina, L.S.; Zakalyukin, R.M.; Sobolev, B.P.; Herrero, P. Modeling of the structure of fluorite-type solid solutions M1–x(Y, Ln)xF2+x from matrix and rare-earth superclusters based on the KY3F10 structure type. Russ. J. Inorg. Chem. 2004, 49, 225–230. [Google Scholar]
- Ranieri, I.M.; Baldochi, S.L.; Santo, A.M.E.; Gomes, L.; Courrol, L.C.; Tarelho, L.V.G.; de Rossi, W.; Berretta, J.R.; Costa, F.E.; Nogueira, G.E.C.; et al. Growth of LiYF4 crystals doped with holmium, erbium and thulium. J. Cryst. Growth 1996, 166, 423–428. [Google Scholar] [CrossRef]
- Devyatkova, K.M.; Ivanova, O.N.; Seiranyan, K.B.; Tamazyan, S.A.; Chernov, S.P. Vacuum ultraviolet properties of a new fluoride matrix. Sov. Phys. Dokl. 1990, 35, 40–41. [Google Scholar]
- Nawata, T.; Inui, Y.; Masada, I.; Nishijima, E.; Satoh, H.; Fukuda, T. High index fluoride materials for 193 nm immersion lithography. In Proceedings of the SPIE 6154 Optical Microlithography XIX, San Jose, CA, USA, 15 March 2006. 61541A. [Google Scholar] [CrossRef]
- Popov, P.A.; Fedorov, P.P.; Semashko, V.V.; Korableva, S.L.; Marisov, M.A.; Gordeev, E.Yu; Reiterov, V.M.; Osiko, V.V. Thermal conductivity of crystals formed by fluorite-like phases in MF–RF3 systems (M = Li, Na, and K, R = Rare Earth). Dokl. Phys. 2009, 54, 221–224. [Google Scholar] [CrossRef]
- Mironov, E.A.; Palashov, O.V.; Voitovich, A.V.; Karimov, D.N.; Ivanov, I.A. Investigation of thermo-optical characteristics of magneto-active crystal Na0.37Tb0.63F2.26. Opt. Lett. 2015, 40, 4919–4922. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.A.; Ivanov, I.A.; Karimov, D.N. Investigation of the thermal conductivity terbium gallium and terbium scandium aluminum garnet crystals. Crystallogr. Rep. 2018, 63, 451–455. [Google Scholar] [CrossRef]
- Karimov, D.N.; Buchinskaya, I.I.; Sorokin, N.I.; Glushkova, T.M.; Chernov, S.P.; Popov, P.A. Growth and some physical properties of congruently melting fluorite solid solutions crystals in the CaF2–SrF2–RF3 (R = La, Ce) systems. Crystallogr. Rep. 2019, 64, 834–840. [Google Scholar] [CrossRef]
- Karimov, D.N.; Buchinskaya, I.I.; Sorokin, N.I.; Sobolev, B.P.; Popov, P.A. Crystal growth and thermal conductivity of the congruently melting solid solution Cd0.77Sr0.23F2. Inorg. Mater. 2019, 55, 495–499. [Google Scholar] [CrossRef]
- Moiseev, N.V.; Popov, P.A.; Fedorov, P.P.; Garibin, E.A.; Reiterov, V.M. Thermodynamic properties of Ca1−xErxF2+x and Ca1−xYbxF2+x heterovalent solid solutions. Inorg. Mater. 2013, 49, 325–328. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Ivanov-Shits, A.K.; Vistin, L.L.; Sobolev, B.P. Anion conductivity of Na0.5–xR0.5+xF2+x (R = Dy–Lu, Y; x = 0.1) single crystals with the fluorite structure. Sov. Phys. Crystallogr. 1992, 37, 217–220. [Google Scholar]
- Jalali, A.A.; Rogers, E.; Stevens, K. Characterization and extinction measurement of potassium terbium fluoride single crystal for high laser power applications. Opt. Lett. 2017, 42, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, B.P.; Sorokin, N.I.; Bolotina, N.B. Nonstoichiometric single crystals M1−xRxF2+x and R1-yMyF3-y (M—Ca, Sr, Ba; R—rare earth elements) as fluorine-conducting solid electrolytes. In Progress in Fluorine Science. Photonic & Electronic Properties of Fluoride Materials; Tressaud, A., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 465–491, Chapter 21. [Google Scholar] [CrossRef]
- Pontonnier, L.; Patrat, G.; Aleonard, S.; Capponi, J.J.; Brunel, M.; de Bergevin, F. An approach to the local arrangement of the fluorine atoms in the anionic conductors with the fluorite structure Na0.5-xY0.5+xF2+2x. Solid State Ionics 1983, 9–10, 549–554. [Google Scholar] [CrossRef]
- Zhurova, E.A.; Maximov, B.A.; Sobolev, B.P.; Simonov, V.I.; Hull, S.; Wilson, C.C. Defect structure of Na0.39Y0.61F2.22 crystals. Crystallogr. Rep. 1997, 42, 238–242. [Google Scholar]
- Otroshchenko, L.P.; Fykin, L.E.; Bystrova, A.A.; Sobolev, B.P. Defect structure of Na0.5-xR0.5+xF2+2x (R = Ho, Yb) solid solutions (fluorite type). Crystallogr. Rep. 2000, 45, 926–929. [Google Scholar] [CrossRef]
- Maximov, B.A.; Solans, J.; Dudka, A.P.; Genkina, E.A.; Bardia-Font, M.; Buchinskaya, I.I.; Loshmanov, A.A.; Golubev, A.M.; Simonov, V.I.; Font-Altaba, M.; et al. Crystal structure of fluorite-based Ba4R3F17 (R = Y, Yb) phases: The ordering of cations and features of anion arrangement. Crystallogr. Rep. 1996, 41, 50–57. [Google Scholar]
- Sulyanova, E.A.; Karimov, D.N.; Sobolev, B.P. Nanostructured crystals of fluorite phases Sr1−xRxF2+x (R are rare-earth elements) and their ordering. 15. Concentration dependence of the defect structure of as grown nonstoichiometric phases Sr1−xRxF2+x (R = Sm, Gd). Crystallogr. Rep. 2019, 64, 873–878. [Google Scholar] [CrossRef]
- Sulyanova, E.A.; Karimov, D.N.; Sobolev, B.P. Nanostructured crystals of fluorite phases Sr1−xRxF2+x (R are rare-earth elements) and their ordering. 16: Defect structure of the nonstoichiometric phases Sr1−xRxF2+x (R = Pr, Tb–Yb) as grown. Crystallogr. Rep. 2020, 65, 560–565. [Google Scholar] [CrossRef]
- Pontonnier, L.; Aleonard, S.; Roux, M.T.; Hammou, A. Propertes electriques des solutions solides a structure fluorine excedentaire en anions Na0.5-xY0.5+xF2+2x. J. Solid State Chem. 1987, 69, 10–18. [Google Scholar] [CrossRef]









| Chemical Formula | KTb3F10 | KY3F10 |
|---|---|---|
| Crystal system, space group | Cubic, | |
| a (Å) | 11.67246 (4) | 11.5384 (1) |
| V (Å3) | 1590.33 (2) | 1536.16 (4) |
| Z | 8 | |
| Crystal size (mm) | 0.1 × 0.1 × 0.07 | 0.21 × 0.2 × 0.07 |
| µ (mm−1 ) | 27.046 | 12.575 |
| Radiation type | Mo Ka, λ = 0.71073 Å | Ag Ka, λ = 0.56087 Å |
| Theta range for data collection (°) | 3.0–37.7 | 2.4–30.7 |
| Limiting indices | −19 ≤ h ≤ 19 −19 ≤ k ≤ 19 −19 ≤ l ≤ 20 | −20 ≤ h ≤ 20 −20 ≤ k ≤ 20 −19 ≤ l ≤ 20 |
| Number of measured, independent and observed [I > 2 σ(I)] reflections | 31006, 264, 260 | 27047, 294, 294 |
| Data/restrains/parameters | 264/0/13 | 294/0/13 |
| Rint | 0.027 | 0.067 |
| R[F2 > σ2(F2 )], wR(F2 ), S | 0.008, 0.028, 1.01 | 0.0137, 0.033, 1.28 |
| Largest diff. peak and hole (e/Å3) | 0.53 and −1.10 | 0.53 and −0.92 |
| Absorption correction | Numerical absorption correction based on gaussian integration over a multifaceted crystal model CrysAlis PRO 1.171.41.95a | |
| Tmin, Tmax | 0.196, 0.349 | 0.138, 0.652 |
| Extinction correction: SHELXL2018/3 | Fc* = kFc[1 + 0.001 × Fc2l3/sin(2q)]−1/4 | |
| Extinction coefficient | 0.000255(18) | 0.0128(4) |
| Parameter | KTb3F10 | KY3F10 | Na0.37Tb0.63F2.26 | Na0.4Y0.6F2.2 |
|---|---|---|---|---|
| A, (SK)/cm | 1.2 × 107 | 4.0 × 108 | 3.6 × 104 [44] | 3.1 × 104 [66] |
| Hσ, eV | 1.16 (550–820 K) | 1.57 (630–825 K) | 0.74 (478–700 K) | 0.80 (380–730 K) |
| σdc(500 K), S/cm | 4.9 × 10−8 | 1.2 × 10−10 | 2.6 × 10−6 | 4.8 × 10−6 |
| k(300 K), W/(mK) | 1.7 1.67 [67] | 3.5 [60] | 1.0 [61] | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimov, D.N.; Buchinskaya, I.I.; Arkharova, N.A.; Ivanova, A.G.; Savelyev, A.G.; Sorokin, N.I.; Popov, P.A. Growth Peculiarities and Properties of KR3F10 (R = Y, Tb) Single Crystals. Crystals 2021, 11, 285. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030285
Karimov DN, Buchinskaya II, Arkharova NA, Ivanova AG, Savelyev AG, Sorokin NI, Popov PA. Growth Peculiarities and Properties of KR3F10 (R = Y, Tb) Single Crystals. Crystals. 2021; 11(3):285. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030285
Chicago/Turabian StyleKarimov, Denis N., Irina I. Buchinskaya, Natalia A. Arkharova, Anna G. Ivanova, Alexander G. Savelyev, Nikolay I. Sorokin, and Pavel A. Popov. 2021. "Growth Peculiarities and Properties of KR3F10 (R = Y, Tb) Single Crystals" Crystals 11, no. 3: 285. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030285