A Cadmium Anionic 1-D Coordination Polymer {[Cd(H2O)6][Cd2(atr)2(μ2-btc)2(H2O)4] 2H2O}n within a 3-D Supramolecular Charge-Assisted Hydrogen-Bonded and π-Stacking Network
Abstract
:1. Introduction
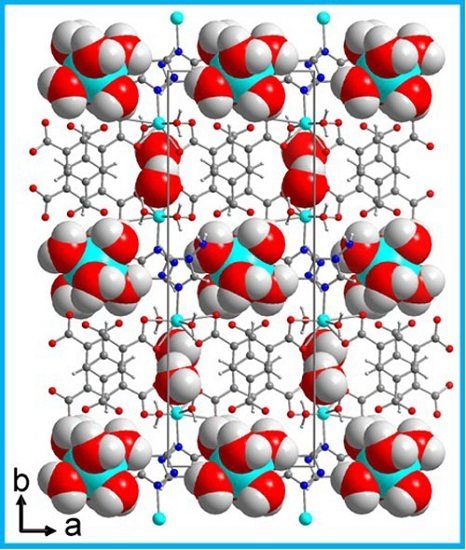
2. Results and Discussion
3. Materials and Methods
Single Crystal X-Ray Structure
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-J.; Yue, Y.-F.; Qian, G.-D.; Chen, B.-L. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion Conductivity and Transport by Porous Coordination Polymers and Metal–Organic Frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Givaja, G.; Amo-Ochoa, P.; Gómez-García, C.J.; Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, D.; Henninger, S.K.; Janiak, C. Multicyle water vapour stability of microporous breathing MOF aluminium isophthalate CAU-10-H. Dalton Trans. 2014, 43, 15300–15304. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Water and methanol adsorption on MOFs for fast cycling heat transformation processes. New J. Chem. 2014, 38, 1846–1852. [Google Scholar] [CrossRef]
- Wang, S.-W.; Yang, L.; Feng, J.-L.; Wu, B.-D.; Zhang, J.-G.; Zhang, T.-L.; Zhou, Z.-N. Synthesis, Crystal Structure, Thermal Decomposition, and Sensitive Properties of Two Novel Energetic Cadmium(II) Complexes Based on 4-Amino-1,2,4-triazole. Z. Anorg. Allg. Chem. 2011, 637, 2215–2222. [Google Scholar] [CrossRef]
- Carpenter, J.A.; Dshemuchadse, J.; Busato, S.; Braeunlich, I.; Pöthig, A.; Caseri, W. Tetrakis(4-amino-1,2,4-triazole)platinum(II) Salts: Syntheses, Crystal Structures, and Properties. Z. Anorg. Allg. Chem. 2014, 640, 724–732. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Cheng, R.-M.; Song, Y.; Li, Y.-Z.; Yu, Z.; Chen, X.-T.; Xue, Z.-L.; You, X.-Z. Syntheses, Structures, and Magnetic Properties of Unusual Nonlinear Polynuclear Copper(II) Complexes Containing Derivatives of 1,2,4-Triazole and Pivalate Ligands. Inorg. Chem. 2005, 44, 8011–8022. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-S.; Lim, J.-H.; Kang, J.-S.; Koh, E.-K.; Hong, C.-S. Triazole-bridged magnetic M(II) assemblies (M = Co, Ni) capped with the end-on terephthalate dianion involving multi-intermolecular contacts. Polyhedron 2007, 26, 4383–4388. [Google Scholar] [CrossRef]
- Yeh, C.-W.; Chang, W.-J.; Suen, M.-C.; Lee, H.-T.; Tsai, H.-A.; Tsou, C.-H. Roles of the anion in the self-assembly of silver(I) complexes containing 4-amino-1,2,4-triazole. Polyhedron 2013, 61, 151–160. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Kuang, X.-F.; Zhao, Z.-G.; Chen, S.-C.; Xie, Y.-M.; Yu, R.-M.; Lu, C.-Z. A series of POM-based hybrid materials with different copper/aminotriazole motifs. Inorg. Chim. Acta 2010, 363, 1236–1242. [Google Scholar] [CrossRef]
- Grosjean, A.; Daro, N.; Kaufmann, B.; Kaiba, A.; Létard, J.-F.; Guionneau, P. The 1-D polymeric structure of the [Fe(NH2trz)](NO)2·nH2O (with n = 2) spin crossover compound proven by single crystal investigations. Chem. Commun. 2011, 47, 12382–12384. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.-T.; Yue, F.; Chen, H.-M.; Liu, G.; Sun, D.-C. Syntheses and structures of 3d–4d heterometallic coordination polymers based on 4-amino-1,2,4-triazole. J. Coord. Chem. 2013, 66, 1889–1896. [Google Scholar] [CrossRef]
- Yang, E.-C.; Zhang, C.-H.; Liu, Z.-Y.; Zhang, N.; Zhao, L.-N.; Zhao, X.-J. Three copper(II) 4-amino-1,2,4-triazole complexes containing differently deprotonated forms of 1,3,5-benzenetricarboxylic acid: Synthesis, structures and magnetism. Polyhedron 2012, 40, 65–71. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhao, W.; Zhang, J.-W.; Lu, Q.-L. Three tetranuclear copper(II) cluster-based complexes constructed from 4-amino-1,2,4-triazole and different aromatic carboxylates: Assembly, structures, electrochemical and magnetic properties. J. Solid State Chem. 2013, 198, 162–168. [Google Scholar] [CrossRef]
- Habib, H.A.; Sanchiz, J.; Janiak, C. Mixed-ligand coordination polymers from 1,2-bis(1,2,4-triazol-4-yl)-ethane and benzene-1,3,5-tricarboxylate: Trinuclear nickel or zinc secondary building units for three dimensional networks with crystal-to-crystal transformation upon dehydration. Dalton Trans. 2008, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Sanchiz, J.; Janiak, C. Magnetic and luminescence properties of Cu(II), Cu(II)4O4 core, and Cd(II) mixed-ligand metal–organic frameworks constructed from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzene-1,3,5-tricarboxylate. Inorg. Chim. Acta 2009, 362, 2452–2460. [Google Scholar] [CrossRef]
- Habib, H.A.; Hoffmann, A.; Höppe, H.A.; Janiak, C. Crystal structures and solid-state CPMAS 13C NMR correlations in luminescent zinc(II) and cadmium(II) mixed-ligand coordination polymers constructed from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzenedicarboxylate. Dalton Trans. 2009, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The concept of mixed organic ligands in metal–organic frameworks: design, tuning and functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-G.; Zhu, X.; Cui, Y.-F.; Liang, N.; Sun, P.-P.; Chen, Q.; Li, B.-L.; Li, H.-Y. Structurally versatile cadmium coordination polymers based on bis(1,2,4-triazol)ethane and rigid aromatic multicarboxylates: Syntheses, structures and properties. CrystEngComm 2014, 16, 1632–1644. [Google Scholar] [CrossRef]
- Habib, H.A.; Janiak, C. Benzene-1,3,5-tricarboxylic acid-1,2-bis-(1,2,4-triazol-4-yl)ethane-water (4/1/2). Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o1199. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Hoffmann, A.; Höppe, H.A.; Steinfeld, G.; Janiak, C. Crystal Structure Solid-State Cross Polarization Magic Angle Spinning 13C NMR Correlation in Luminescent d10 Metal-Organic Frameworks Constructed with the 1,2-Bis(1,2,4-triazol-4-yl)ethane Ligand. Inorg. Chem. 2009, 48, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1970. [Google Scholar]
- Shi, Z.; Li, G.; Wang, L.; Gao, L.; Chen, X.; Hua, J.; Feng, S. Two Three-Dimensional Metal−Organic Frameworks from Secondary Building Units of Zn8(OH)4(O2C−)12 and Zn2((OH)(O2C−)3: [Zn2(OH)(btc)]2(4,4′-bipy) and Zn2(OH)(btc)(pipe). Cryst. Growth Des. 2004, 4, 25–27. [Google Scholar] [CrossRef]
- Dai, J.-C.; Wu, X.-T.; Fu, Z.-Y.; cui, C.-P.; Hu, S.-M.; Du, W.-X.; Wu, L.-M.; Zhang, H.-H.; Sun, R.-Q. Synthesis, Structure, and Fluorescence of the Novel Cadmium(II)−Trimesate Coordination Polymers with Different Coordination Architectures. Inorg. Chem. 2002, 41, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Slangen, P.M.; van Koningsbruggen, P.J.; Goubitz, K.; Haasnoot, J.G.; Reedijk, J. Isotropic Magnetic Exchange Interaction through Double µ-1,2,4-Triazolato-N1,N2 Bridges: X-ray Crystal Structure, Magnetic Properties, and EPR Study of Bis(µ-3-pyridin-2-yl-1,2,4-triazolato-N′,N1,N2)(sulfato-O)aquacopper(II)-diaquacopper(II) Trihydrate. Inorg. Chem. 1994, 33, 1121–1126. [Google Scholar] [CrossRef]
- Stiefel, E.I.; Brown, G.F. Detailed nature of the six-coordinate polyhedra in tris(bidentate ligand) complexes. Inorg. Chem. 1972, 11, 434–436. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, A.; Wu, B.; Lassahn, P.-G.; Janiak, C. Polymethylene spacer regulated structural divergence in cadmium complexes: Unusual trigonal prismatic and severely distorted octahedral coordination. Polyhedron 2005, 24, 593–599. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Ameur, I.; Dkhili, S.; Sbihi, H.; Besbes-Hentati, S.; Rzaigui, M.; Abid, S. A Cadmium Coordination Polymer Based on Phosphate Clusters: Synthesis, Crystal Structure, Electrochemical Investigation and Antibacterial Activity. J. Cluster Sci. 2016. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Chen, Y.; Kou, X.-Y. A novel one-dimensional tubular cadmium(II) coordination polymer based on 5-(carboxylatomethoxy)benzene-1,3-dicarboxylate containing 4-aminopyridinium ions. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Al-Deyab, S.S.; Rzaigui, M. The one-dimensional coordination polymer poly[tetrakis[(4-chloro-phenyl)methanaminium] [cadmate-μ-cyclohexaphosphorato]]. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m1549–m1550. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Ward, M.D. Design of crystalline molecular networks with charge-assisted hydrogen bonds. Chem. Commun. 2005, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Heering, C.; Nateghi, B.; Janiak, C. Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid. Crystals 2016, 6, 22. [Google Scholar] [CrossRef]
- Chamayou, A.-C.; Neelakantan, M.A.; Thalamuthu, S.; Janiak, C. The first vitamin B6 zinc complex, pyridoxinato-zinc acetate: A 1D coordination polymer with polar packing through strong inter-chain hydrogen bonding. Inorg. Chim. Acta 2011, 365, 447–450. [Google Scholar] [CrossRef]
- Drašković, B.M.; Bogdanović, G.A.; Neelakantan, M.A.; Chamayou, A.-C.; Thalamuthu, S.; Avadhut, Y.S.; Schmedt auf der Günne, J.; Banerjee, S.; Janiak, C. N-o-Vanillylidene-L-histidine: Experimental charge density analysis of a double zwitterionic amino acid Schiff-base compound. Cryst. Growth Des. 2010, 10, 1665–1676. [Google Scholar] [CrossRef]
- Dorn, T.; Chamayou, A.-C.; Janiak, C. Hydrophilic interior between hydrophobic regions in inverse bilayer structures of cation–1,1′-binaphthalene-2,2′-diyl phosphate salts. New J. Chem. 2006, 30, 156–167. [Google Scholar] [CrossRef]
- Maclaren, J.K.; Sanchiz, J.; Gili, P.; Janiak, C. Hydrophobic-exterior layer structures and magnetic properties of trinuclear copper complexes with chiral amino alcoholate ligands. New J. Chem. 2012, 36, 1596–1609. [Google Scholar] [CrossRef]
- Naik, A.; Marchand-Brynaert, J.; Garcia, Y. A Simplified Approach to N-and N,N′-Linked 1,2,4-Triazoles by Transamination. Synthesis 2008, 1, 149–154. [Google Scholar]
- Bartlett, R.K.; Humphrey, I.R. Transaminations of NN-dimethylformamide azine. J. Chem. Soc. C 1967, 1664–1666. [Google Scholar] [CrossRef]
- APEX2. SAINT, Data Reduction and Frame Integration Program for the CCD Area-Detector System, Bruker Analytical X-ray Systems, Data Collection program for the CCD Area-Detector System; Brucker: Madison, WI, USA, 1997–2006. [Google Scholar]
- Sheldrick, G. SADABS: Area-Detector Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Crystal and Molecular Structure Visualization, Crystal Impact—K. In Diamond, version 3.2; Brandenburg, H., Ed.; Putz Gbr: Bonn, Germany, 2009. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]



| Cd1–N1 | 2.253(6) | O8–Cd1–O7 | 171.88(16) |
| Cd1–O1 | 2.412(5) | N1–Cd1–O2 | 142.95(16) |
| Cd1–O2 | 2.355(5) | O5 i–Cd1–O2 | 84.36(16) |
| Cd1–O5 i | 2.258(5) | O8–Cd1–O2 | 89.48(18) |
| Cd1–O6 i | 2.766(5) | O7–Cd1–O2 | 82.40(17) |
| Cd1–O7 | 2.325(5) | N1–Cd1–O1 | 88.09(17) |
| Cd1–O8 | 2.302(5) | O5 i–Cd1–O1 | 139.22(16) |
| – | – | O8–Cd1–O1 | 90.35(17) |
| Cd2–O9 | 2.275(5) | O7–Cd1–O1 | 84.81(17) |
| Cd2–O10 | 2.304(6) | O2–Cd1–O1 | 54.86(15) |
| Cd2–O11 | 2.266(5) | – | – |
| – | – | O9–Cd2–O10 | 86.65(18) |
| N1–Cd1–O5 i | 132.67(18) | O9–Cd2–O10 ii | 93.35(18) |
| N1–Cd1–O8 | 90.45(19) | O10-Cd2–O11 | 86.1(2) |
| O5 i–Cd1–O8 | 88.47(18) | O10–Cd2–O11 ii | 93.9(2) |
| N1–Cd1–O7 | 95.88(18) | O9–Cd2–O11 | 87.50(19) |
| O5 i–Cd1–O7 | 90.94(17) | O9–Cd2–O11 ii | 92.50(19) |
| D-H···A | D-H [Å] | H···A [Å] | D···A [Å] | D-H···A [°] | Symmetry Transformations |
|---|---|---|---|---|---|
| O7–H7A···N3 iv | 0.95 | 2.33 | 3.280(7) | 176 | iv = –x + 2, −y + 1, −z + 1 |
| O7–H7A···N4 iv | 0.95 | 2.33 | 3.138(8) | 143 | iv = –x + 2, −y + 1, −z + 1 |
| O7–H7B···O12 i | 0.95 | 2.07 | 2.684(7) | 121 | i = x + 1, y, z |
| O8–H8A···O11 v | 0.95 | 2.41 | 3.284(7) | 152 | v = x + 1, y, z + 1 |
| O8–H8B···O12 v | 0.95 | 2.05 | 2.695(7) | 124 | v = x + 1, y, z + 1 |
| O9–H9A···O3 vi | 0.92(4) | 1.88(5) | 2.785(7) | 167(8) | vi = −x + 1, y + 1/2, −z + 3/2 |
| O9–H9B···N2 ii | 0.89(9) | 2.02(9) | 2.897(8) | 171(8) | ii = −x + 1, −y + 1, −z + 1 |
| O10–H10A···O6 | 0.95(9) | 1.84(9) | 2.742(7) | 158(7) | – |
| O10–H10B···N4 vii | 0.98(5) | 1.91(6) | 2.803(8) | 151(7) | vii = −x + 2, −y + 1, −z + 2 |
| O11–H11A···O6 | 1.00(9) | 1.93(9) | 2.906(8) | 167(8) | – |
| O11–H11B···O4 viii | 0.92(5) | 1.78(6) | 2.641(7) | 155(8) | viii = x, −y + 1/2, z − 1/2 |
| O12–H12A···O2 x | 0.89(5) | 1.84(6) | 2.661(7) | 152(8) | x = x − 1, –y + 1/2, z − 1/2 |
| O12–H12B···O5 | 0.92 | 1.85 | 2.763(7) | 172 | – |
| N4–H4A···O3 ix | 0.85(8) | 2.24(8) | 3.081(8) | 170(7) | ix = 2 − x, y + 1/2, −z + 3/2 |
| N4–H4B···O1 vii | 0.83(4) | 2.04(5) | 2.857(8) | 166(8) | vii = −x + 2, −y + 1, −z + 2 |
| C10–H10···O10 i | 0.95 | 2.54 | 3.381(8) | 147 | i = x + 1, y, z |
| Chemical Formula | C22H38Cd3N8O24 |
|---|---|
| Mr | 1131.80 |
| Crystal system, space group | Monoclinic, P 21/c |
| Temperature (K) | 100(2) |
| a (Å) | 10.1435(18) |
| b (Å) | 26.471(5) |
| c (Å) | 7.0263(13) |
| β (°) | 106.213(13) |
| V (Å3) | 1811.6(6) |
| Z | 2 |
| Density (calculated), Mg/m3 | 2.082 |
| Absorpt. coefficient, μ (mm–1) | 1.850 |
| Crystal size (mm) | 0.3 × 0.3 × 0.1 |
| F(000) | 1124 |
| Theta range for data collection, (°) | 2.09–25.34 |
| h, k, l ranges | ±12, ±31, ±8 |
| Reflections collected | 15909 |
| Independent reflections | 3279 [R(int) = 0.1114] |
| Completeness to theta 25.34° | 98.9% |
| Data/restraints/parameters | 3279/5/286 |
| Final R indices [I > 2sigma(I)] a | R1 = 0.0519, wR2 = 0.1284 |
| R indices (all data) a | R1 = 0.0734, wR2 = 0.1418 |
| Goodness-of-fit on F2 b | 1.020 |
| Weighting scheme w; a/b c | 0.0521/0.000 |
| Δρmax, Δρmin (e Å–3) d | 0.30, −0.34 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahli, A.; Köc, Ü.; Elshaarawy, R.F.M.; Kautz, A.C.; Janiak, C. A Cadmium Anionic 1-D Coordination Polymer {[Cd(H2O)6][Cd2(atr)2(μ2-btc)2(H2O)4] 2H2O}n within a 3-D Supramolecular Charge-Assisted Hydrogen-Bonded and π-Stacking Network. Crystals 2016, 6, 23. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6030023
Tahli A, Köc Ü, Elshaarawy RFM, Kautz AC, Janiak C. A Cadmium Anionic 1-D Coordination Polymer {[Cd(H2O)6][Cd2(atr)2(μ2-btc)2(H2O)4] 2H2O}n within a 3-D Supramolecular Charge-Assisted Hydrogen-Bonded and π-Stacking Network. Crystals. 2016; 6(3):23. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6030023
Chicago/Turabian StyleTahli, Anas, Ümit Köc, Reda F. M. Elshaarawy, Anna Christin Kautz, and Christoph Janiak. 2016. "A Cadmium Anionic 1-D Coordination Polymer {[Cd(H2O)6][Cd2(atr)2(μ2-btc)2(H2O)4] 2H2O}n within a 3-D Supramolecular Charge-Assisted Hydrogen-Bonded and π-Stacking Network" Crystals 6, no. 3: 23. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6030023