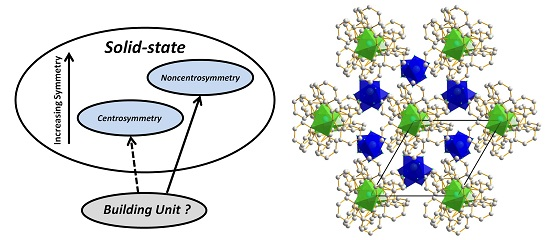
Packing of Helices: Is Chirality the Highest Crystallographic Symmetry?
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pasteur, L. Sur les relations qui peuvent exister entre la forme criastalline, la composition chimique, et le sens de la polarization rotatoire. Ann. Chim. Phys. 1848, 24, 442–459. (In French) [Google Scholar]
- Pasteur, L. Recherches sur les propriétés spécifiques des deux acides qui composent l’acide racémique. impr. Bachelier. Ann. Chim. Phys. 1850, 28, 56–99. (In French) [Google Scholar]
- Flack, H.D. Chiral and Achiral Crystal Structures. Helv. Chim. Acta 2003, 86, 905–921. [Google Scholar]
- Donakowski, M.D.; Gautier, R.; Yeon, J.; Moore, D.T.; Nino, J.C.; Halasyamani, P.S.; Poeppelmeier, K.R. The Role of Polar, Lamdba (Λ)-Shaped Building Units in Noncentrosymmetric Inorganic Structures. J. Am. Chem. Soc. 2012, 134, 7679–7689. [Google Scholar]
- Gautier, R.; Poeppelmeier, K.R. Preservation of Chirality and Polarity between Chiral and Polar Building Units in the Solid State. Inorg. Chem. 2012, 51, 10613–10618. [Google Scholar]
- Maggard, P.A.; Kopf, A.L.; Stern, C.L.; Poeppelmeier, K.R. Probing helix formation in chains of vertex-linked octahedra. CrystEngComm 2004, 6, 452–457. [Google Scholar] [CrossRef]
- Gautier, R.; Norquist, A.J.; Poeppelmeier, K.R. From Racemic Units to Polar Materials. Cryst. Growth Des. 2012, 12, 6267–6271. [Google Scholar] [CrossRef]
- Gautier, R.; Klingsporn, J.M.; Van Duyne, R.P.; Poeppelmeier, K.R. Optical activity from racemates. Nat. Mater. 2016, 15, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, N.; Ramakumar, S.; Viswamitra, M.A. Space-group frequencies of proteins and of organic compounds with more than one formula unit in the asymmetric unit. Acta Crystallogr. A 1990, 46, 725–730. [Google Scholar] [CrossRef]
- Steed, J.W. Should solid-state molecular packing have to obey the rules of crystallographic symmetry? CrystEngComm 2003, 5, 169–179. [Google Scholar] [CrossRef]
- Sharma, V.; Crne, M.; Park, J.O.; Srinivasarao, M. Structural Origin of Circularly Polarized Iridescence in Jeweled Beetles. Science 2009, 325, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-J.; Oh, J.-W.; Kwak, K.; Lee, B.Y.; Meyer, J.; Wang, E.; Hexemer, A.; Lee, S.-W. Biomimetic self-templating supramolecular structures. Nature 2011, 478, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Hallé, M.A.; Dumoulin, H.; Pieranski, P. Structure of the cholesteric focal conic domains at the free surface. Phys. Rev. E 1996, 54, 3771–3782. [Google Scholar] [CrossRef]
- Chothia, C.; Levitt, M.; Richardson, D. Helix to helix packing in proteins. J. Mol. Biol. 1981, 145, 215–250. [Google Scholar] [CrossRef]
- Boncheva, M.; Bruzewicz, D.A.; Whitesides, G.M. Formation of chiral, three-dimensional aggregates by self-assembly of helical components. Langmuir 2003, 19, 6066–6071. [Google Scholar] [CrossRef]
- Meille, S.V.; Allegra, G. Chiral crystallization of helical polymers. Macromolecules 1995, 28, 7764–7769. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Lv, H.; Li, T.; Haso, F.; Hu, L.; Zhang, B.; Bacsa, J.; Wei, Y.; Gao, Y.; et al. Chiral recognition and selection during the self-assembly process of protein-mimic macroanions. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Maggard, P.A.; Stern, C.L.; Poeppelmeier, K.R. Understanding the Role of Helical Chains in the Formation of Noncentrosymmetric Solids. J. Am. Chem. Soc. 2001, 123, 7742–7743. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T. Int. Tables Crystallogr; D. Reidel: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Breu, J.; Domel, H.; Stoll, A. Racemic Compound Formation versus Conglomerate Formation with [M (bpy) 3](PF6) 2 (M = Ni, Zn, Ru); Molecular and Crystal Structures. Eur. J. Inorg. Chem. 2000, 2000, 2401–2408. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.-Y.; Huang, W. Tris (2, 2′-bipyridyl-κ2N, N′) copper (II) hexafluoridophosphate. Acta Crystallogr. Sect. E 2007, 63, m835–m836. [Google Scholar] [CrossRef]
- Kundu, N.; Mandal, D.; Chaudhury, M.; Tiekink, E.R.T. Luminescence characteristics and X-ray crystal structure of [Cd (bipy) 3][PF6] 2 (bipy = 2, 2′-bipyridine). Appl. Organomet. Chem. 2005, 19, 1268–1270. [Google Scholar] [CrossRef]
- He, C.; Zhao, Y.; Guo, D.; Lin, Z.; Duan, C. Chirality transfer through helical motifs in coordination compounds. Eur. J. Inorg. Chem. 2007, 2007, 3451–3463. [Google Scholar] [CrossRef]
- Ghazzali, M.; Langer, V.; Öhrström, L. The role of intermolecular interactions in the assemblies of FeII and CoII tetrakis-isothiocyanatometalates with tris(1,10-phenanthroline)-RuII: Crystal structures of two dual-metal assemblies featuring octahedral cationic and tetrahedral anionic modules. J. Solid State Chem. 2008, 181, 2191–2198. [Google Scholar] [CrossRef]
- Matta, C.F.; Castillo, N.; Boyd, R.J. Extended Weak Bonding Interactions in DNA: π-Stacking (Base−Base), Base−Backbone, and Backbone−Backbone Interactions. J. Phys. Chem. B 2005, 110, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-D.; Jiang, L.; Feng, X.-L.; Lu, T.-B. Constructions of 1D helical chains with left-handed/right-handed helicity: A correlation between the helicity of 1D chains and the chirality of building blocks. Dalton Trans. 2009, 6802–6808. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautier, R.; Poeppelmeier, K.R. Packing of Helices: Is Chirality the Highest Crystallographic Symmetry? Crystals 2016, 6, 106. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090106
Gautier R, Poeppelmeier KR. Packing of Helices: Is Chirality the Highest Crystallographic Symmetry? Crystals. 2016; 6(9):106. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090106
Chicago/Turabian StyleGautier, Romain, and Kenneth R. Poeppelmeier. 2016. "Packing of Helices: Is Chirality the Highest Crystallographic Symmetry?" Crystals 6, no. 9: 106. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090106