Synthesis of Novel p-tert-Butylcalix[4]arene Derivative: Structural Characterization of a Methanol Inclusion Compound
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Characterization
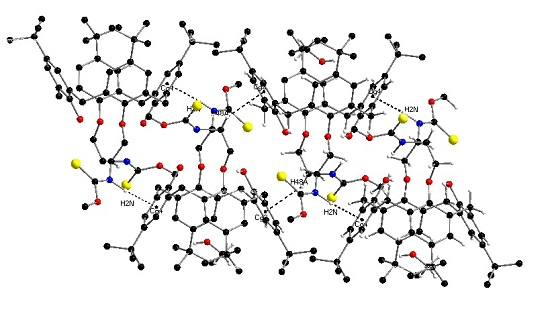
2.2. Crystal Structure
3. Materials and Methods
3.1. Sample Preparation
3.2. Single Crystal X-ray Diffraction
| Empirical Formula | C52H70N2O6S2, CH4O |
|---|---|
| Formula weight | 915.31 |
| Crystal size (mm3) | 0.4 × 0.3 × 0.09 |
| Crystal system, Space group | Triclinic, P-1 |
| a (Å) | 12.571(3) |
| b (Å) | 14.759(3) |
| c (Å) | 16.835(3) |
| α (°) | 67.08(3) |
| β (°) | 68.96(3) |
| γ (°) | 78.37(3) |
| Volume (Å3) | 2678.1(12) |
| Z | 2 |
| Temperature | 298 K |
| ρ calculated/g·cm−3 | 1.135 |
| μmm−1 | 0.148 |
| hkl range | −13/14, −17/17,−20/20 |
| θ range (°) | 3.5–25.0 |
| Reflections collected | 35842 |
| Unique reflections (Rint) | 9405 [0.111] |
| Observed data (I > 2σ (I)) | 9405 |
| R [(F2 > 2σ (F2)) | 0.1124 |
| wR(F2) | 0.2077 |
| S = GooF | 1.34 |
| Parameters | 603 |
| Δρmax, Δρmin | 0.77 e Å−3, −0.70 e Å−3 |
3.3. Raman and Mass Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asfari, M.-Z.; Böhmer, V.; Harrowfield, J.; Vicens, J. Calixarenes 2001; Springer: Berlin, Germany, 2001; p. 577. [Google Scholar]
- Zhang, W.-C.; Huang, Z.-T. Synthesis of 4-tert-butylcalix[4]arenes Bearing two Schiff-Base units at the Lower Rim. Synthesis 1997, 9, 1073–1076. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, M.H.; Mutihac, L.; Vicens, J.; Kim, J.S. Host-guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 2012, 41, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D.; Stoddart, J.F.; Aida, T. Calixarenes Revisited; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 75–77. [Google Scholar]
- Ozmen, M.; Ozbek, Z.; Bayrakci, M.; Ertul, S.; Ersoz, M. Applied Surface Science Preparation of Langmuir–Blodgett thin films of calix[6]arenes and p-tert butyl group effect on their gas sensing properties. Appl. Surf. Sci. 2015, 359, 364–371. [Google Scholar] [CrossRef]
- Jin, C.; Fukuda, M.; Wu, C. A pyrene-armed hexahomotrioxacalix[3]arene as a multi-sensor via synergistic and demetallation effects. Tetrahedron 2015, 71, 9593–9597. [Google Scholar] [CrossRef]
- Mokhtari, B.; Pourabdollah, K. Applications of calixarene nano-baskets in pharmacology. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 1–15. [Google Scholar] [CrossRef]
- Bozkurt, S.; Yilmaz, M.; Sirit, A. Chiral Calix[4]arenes Bearing Amino Alcohol Functionality as Membrane Carriers for Transport of Chiral Amino Acid Methylesters and Mandelic Acid. Chirality 2012, 136, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.; Ehlinger, N.; Lecocq, S.; Dumazet, I.; Lamartine, R. Crystal Structures of Two Calix[10]arenes Complexed with Neutral Molecules. J. Incl. Phenom. Macrocycl. Chem. 2001, 82282, 273–276. [Google Scholar] [CrossRef]
- McKervey, M.A.; Seward, E.M.; Ferguson, G.; Ruhl, B. Molecular Receptor. Synthesis and X-ray Crystal Structure of a Calix[4]arene tetracarbonate-acetonitrile (1:1) Clathrate. J. Org. Chem. 1986, 51, 3581–3584. [Google Scholar] [CrossRef]
- Huang, G.; Jiang, D.; Wang, D. Intercalation of thiacalix[4]arene anion via calcined/restored reaction into LDH and efficient heavy metal capture. J. Mol. Liq. 2016, 220, 346–353. [Google Scholar] [CrossRef]
- Arduini, A.; Demuru, D.; Pochini, A.; Secchi, A. Recognition of quaternary ammonium cations by calix[4]arene derivatives supported on gold nanoparticles. Chem. Commun. 2005, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-T.; Liu, Y.; Zhang, H.-Y. Supramolecular architecture of self-adhesive calix[4]arene thiourea derivative by hydrogen bonding and π–π interactions. J. Mol. Struct. 2004, 691, 25–31. [Google Scholar] [CrossRef]
- Collins, E.; Harrisc, S.J.; Owens, M.; Ferguson, G.; Estate, I.; McKervey, M.A. Chemically Modified Calix[4]arenes. Regioselective Synthesis of 1,3-(Distal) Derivatives and Related Compounds. X-ray Crystal Structure of a Diphenol-Dinitrile. J. Chem. Soc. Perkin Trans. 1991, 3, 2–3. [Google Scholar] [CrossRef]
- Berstein, J.; Davis, R.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Boeyens, J. The conformation of six-membered rings. J. Cryst. Mol. Struct. 1978, 8, 317–320. [Google Scholar] [CrossRef]
- Fernandez, J.M.G.; Mellet, C.O.; Fuentes, J. Chiral2-Thioxotetrahydro-1,3-O,N-heterocycles and Bicyclic Oxazine-2-t hionest from Carbohydrates. 2. Stereocontrolled Synthesis of Oxazolidine Pseudo-C-nucleosides and bicyclic oxazine-2-thiones. J. Org. Chem. 1993, 58, 5192–5199. [Google Scholar] [CrossRef]
- Torres-pinedo, A.; Saitz, C.; Santoyo-Gonzalez, F. An Efficient Synthesis of Bis(calix[4]arenes), Bis (crown ether)-Substituted Calix[4]arenes, Aza-Crown Calix[4]arenes, and Thiaza-Crown Calix[4]arenes. Eur. J. Org. Chem. 2000, 21, 3587–3593. [Google Scholar]
- Katritzky, A.R.; Bernard, M.K.; Long, Q.-H. The reactions of some alkoxycarbonylisothiocyanates with alcohols, phenols and amines. Org. Prep. Proced. Int. 1993, 25, 83–90. [Google Scholar] [CrossRef]
- Bruker. SMART, SAINTPLUS V6.02, SHELXTL V6.10 and SADABS; Bruker Analytical X-ray Instruments Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Sheldrick, G.M. SHELXL-97. Program for the Refinement of Crystal Structures; University of Göttingen: Stuttgart, Germany, 1997. [Google Scholar]
- Brandenburg, K. DIAMOND. Visual Crystal Structure Information System; Version 2.1e; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]







| Bond Length | Angles | ||
|---|---|---|---|
| C1-C2 | 1.374(8) | C25-O1-C46 | 113.9(4) |
| S1-C49 | 1.600(11) | C27-O3-C45 | 113.7(4) |
| S2-C51 | 1.624(7) | C49-O50-C67 | 123.1(12) |
| O50-C49 | 1.434(17) | C51-O52-C69 | 119.1(7) |
| O52-C51 | 1.335(10) | C48-N1-C51 | 124.8(6) |
| O50-C67 | 1.32(2) | C47-N2-C49 | 124.9(8) |
| O52-C69 | 1.430(12) | S1-C49-O50 | 120.7(10) |
| N1-C48 | 1.441(10) | S1-C49-N2 | 126.3(10) |
| N2-C47 | 1.411(11) | O50-C49-N2 | 113.0(9) |
| D-X···A | d(D-X) | d(X···A) | d(D···A) | <(DXA) |
|---|---|---|---|---|
| C67-H67C···O50 i | 0.97(4) | 2.26(4) | 3.222(2) | 176(3) |
| N1-H1N···O4 ii | 1.17(11) | 2.06(10) | 3.193(7) | 161(8) |
| O2-H2···O3 ii | 0.8200 | 1.93 | 2.746(6) | 175.00 |
| O4-H4···O1 ii | 0.8200 | 1.96 | 2.778(6) | 179.00 |
| C67-H67A···S1 ii | 0.9600 | 2.45 | 2.98(2) | 115.00 |
| C69-H69A···S2 ii | 0.9600 | 2.46 | 2.983(11) | 114.00 |
| C1S-H1S1···Cg2 ii | 0.9600 | 2.72 | 3.61(2) | 73.0 |
| N2-H2N···Cg4 iii | 1.13(7) | 2.17(7) | 3.290(8) | 79.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moris, S.; Galdámez, A.; Jara, P.; Saitz-Barria, C. Synthesis of Novel p-tert-Butylcalix[4]arene Derivative: Structural Characterization of a Methanol Inclusion Compound. Crystals 2016, 6, 114. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090114
Moris S, Galdámez A, Jara P, Saitz-Barria C. Synthesis of Novel p-tert-Butylcalix[4]arene Derivative: Structural Characterization of a Methanol Inclusion Compound. Crystals. 2016; 6(9):114. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090114
Chicago/Turabian StyleMoris, Silvana, Antonio Galdámez, Paul Jara, and Claudio Saitz-Barria. 2016. "Synthesis of Novel p-tert-Butylcalix[4]arene Derivative: Structural Characterization of a Methanol Inclusion Compound" Crystals 6, no. 9: 114. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst6090114